Deep sequencing questions role of imprinted genes in autism
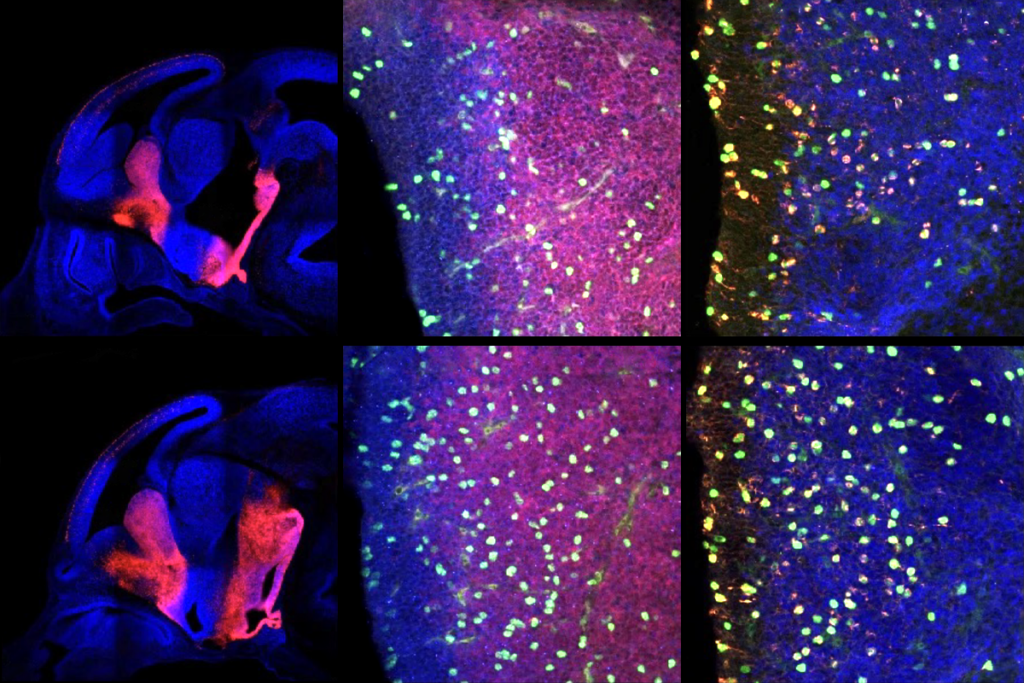
The mouse brain has more than 1,300 regions for which the copy from one parent is expressed more often than the one from the other parent, according to two studies published today in Science. These so-called imprinted genes have been proposed to cause some cases of autism, but the researchers say their findings do not support that theory.
The mouse brain has more than 1,300 regions for which the copy from one parent is expressed more often than the one from the other parent, according to two studies published today in Science1,2. These so-called imprinted genes have been proposed to cause some cases of autism, but the researchers say their findings do not support that theory.
The identified regions include 824 genes, of which 604 have known human counterparts. Statistical models predicted the existence of only a few hundred imprinted genes. Scientists had identified fewer than 100, and tied them to a number of conditions ranging from embryonic growth to autism and schizophrenia.
Using sophisticated sequencing methods, the new work gives the first high-resolution picture of imprinting across the genome.
Experts applaud the studies for their thoroughness, but caution that imprinting patterns in humans might be different than in mice.
“[The new work] is a really important milestone for saying we need to do this for humans,” says Bernie Crespi, professor of evolutionary biology at Simon Fraser University in British Columbia, who was not involved in the new studies. “If we do that, we might find a good chunk of the missing heritability of any number of psychiatric conditions.”
Some autism-related disorders are clearly affected by imprinting. For example, Angelman syndrome, characterized by developmental delay and a happy demeanor, and Prader-Willi syndrome, marked by learning disabilities and compulsive eating, both stem from deletions or duplications in imprinted genes on chromosome 15.
In 2006, Crespi proposed that glitches in imprinted genes also contribute to autism3, and the idea has since gained some traction. He points out that several of the imprinted genes in the new study — including GABRB3, SHANK2 and APBA2 — have been linked to autism.
But the study’s researchers are more skeptical.
“All of these grand theories of imprinting and autism I think are not validated by our data,” says Catherine Dulac, professor of molecular and cellular biology at Harvard University and lead investigator of both studies.
The long list of imprinted genes includes some related to mental retardation, but none that are strong candidates for autism, she says. “On the other hand, I think one can have a completely fresh look at the phenomenon of autism based on the repertoire of the genes that we identified.”
The new studies include many surprises. For example, imprinted genes are not expressed uniformly in all brain areas.
“This work shows that there’s really quite a bit of stage and tissue specificity [in imprinting],” Crespi says. Researchers will need to investigate imprinted genes in other tissues, he adds. “The list is not by any means complete.”
The new studies also found that 347 genes show different imprinting patterns in males and females.
This sex specificity could explain the “otherwise rather curious” observations that genetic mutations are more likely in boys with autism than in girls with the disorder, notes David Skuse, professor of behavioral sciences at University College London.
Autism imprints:
Most genes express two copies, one from each parent. In the case of a few genes, however, one of the copies is silenced in the egg or sperm by the addition of a methyl group to the DNA4. Researchers still don’t know much about why this happens.
The prevailing theory holds that imprinting can be explained by an evolutionary competition between the genes from each parent5. Genes that induce fetal growth, for example, produce more robust offspring, helping the father pass on his genes at no personal cost. But large fetuses require more resources from the mother, which may hinder her future reproduction. In this case, it would make evolutionary sense for the mother to silence her copy of the gene.
One of the first known imprinted genes — insulin-like growth factor 2 (IGF2) — fits this theory perfectly: it promotes growth during gestation, and only the copy inherited from the father is expressed.
Applying these ideas to autism and related disorders, Crespi and others have argued that because children with autism demand extra time and resources from the mother, the disorder could stem from large defects in genes whose maternal copies are silenced6.
This theory is supported by the observation that children with autism have larger heads and, according to some reports7, higher birth weights. Some people with the disorder also show abnormally high expression of IGF28. People with Beckwith-Wiedemann syndrome — in which both copies of IGF2 are expressed — are more likely to have autism than are their healthy peers9.
In the new work, researchers first used the Allen Brain Atlas, an interactive database of mouse gene expression, to map known imprinted genes. They found that imprinted genes tend to appear in regions that would be predicted by the parental competition theory, such as parts of the hypothalamus that control feeding behaviors. In contrast, non-imprinted control genes are expressed mostly in the cortex, or the outer layers of the brain.
The team then searched for new imprinted genes across the mouse genome in three types of brain tissue — the adult cortex, adult hypothalamus and embryonic whole brain.
Intriguingly, genes from the mother are expressed more often in the embryonic brain, whereas ones from the father are more common in the adult brain, the study found. Still, interpreting these patterns is difficult for behavioral genes that have many genetic or environmental interactions, says Jon Wilkins, a professor at the Santa Fe Institute who studies the evolution of imprinting.
Dulac’s group plans to perform high-power searches of imprinted genes in additional brain regions, as well as compare how imprinting patterns are affected by environmental influences, such as stress.
“What is the imprinting of each individual gene doing to the whole of brain function? That’s what will be really difficult to sort out,” she says.
References:
-
Gregg C. et al. Science Express Epub ahead of print (2010) Abstract
-
Gregg C. et al. Science Express Epub ahead of print (2010) Abstract
-
Badcock, C. and B. Crespi J. Evol. Biol. 19, 1007–1032 (2006) PubMed
-
Reik W. and J. Walter Nat. Rev. Genet. 2, 21-32 (2001) PubMed
-
Haig D. Annu. Rev. Genet. 38, 553-585 (2004) PubMed
-
Crespi B. and C. Badcock Behav. Brain Sci. 31, 241-261 (2008) PubMed
-
Mraz K.D. et al. J. Child Neurol. 22, 700-13 (2007) PubMed
-
Mills J.L. et al. Clin. Endocrinol. (Oxf) 67, 230-237 (2007) PubMed
-
Kent L. et al. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 1295-1297 (2008) PubMed
Recommended reading
PTEN problems underscore autism connection to excess brain fluid

Autism traits, mental health conditions interact in sex-dependent ways in early development

New tool may help untangle downstream effects of autism-linked genes
Explore more from The Transmitter

Newly awarded NIH grants for neuroscience lag 77 percent behind previous nine-year average

