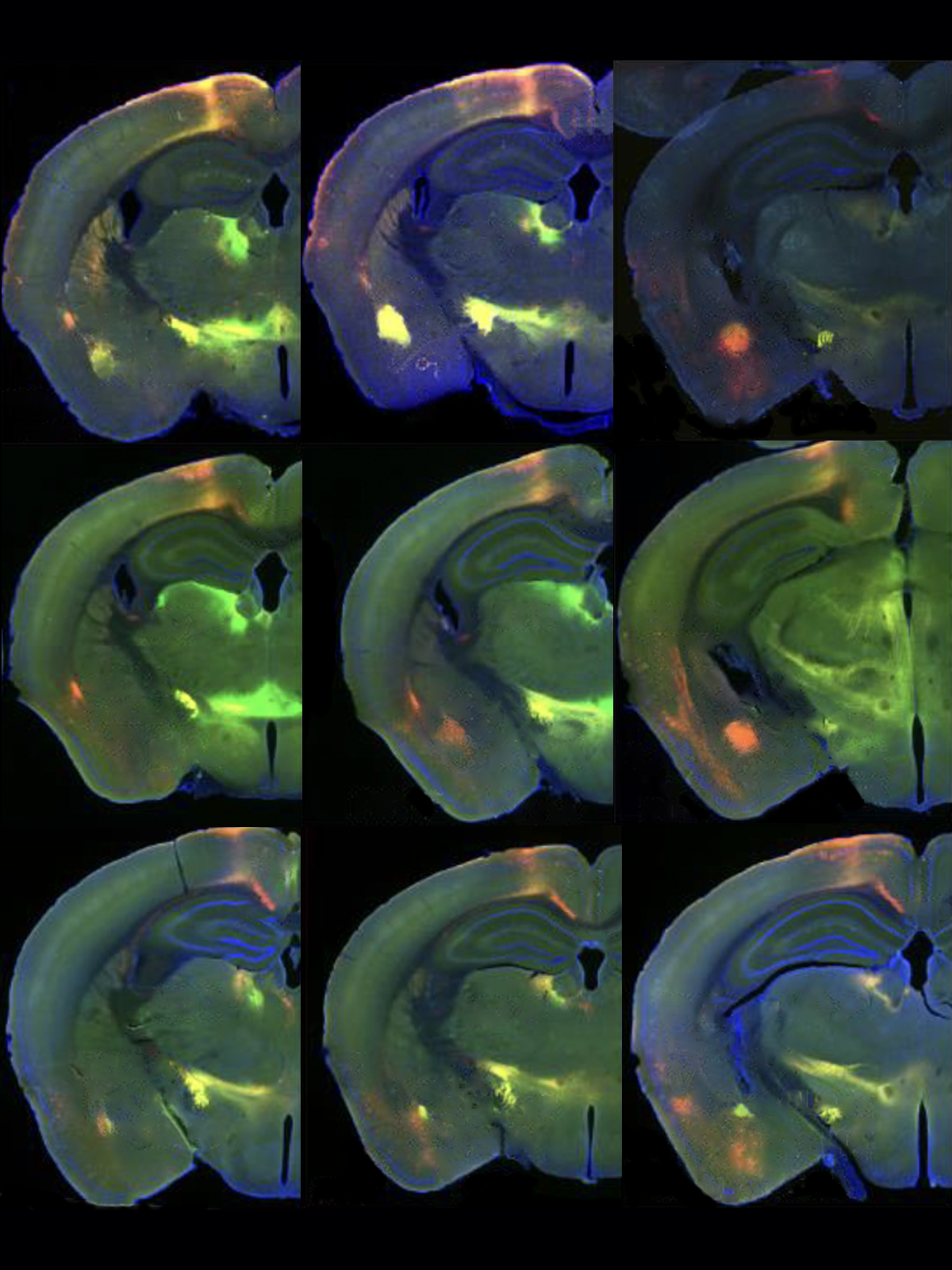
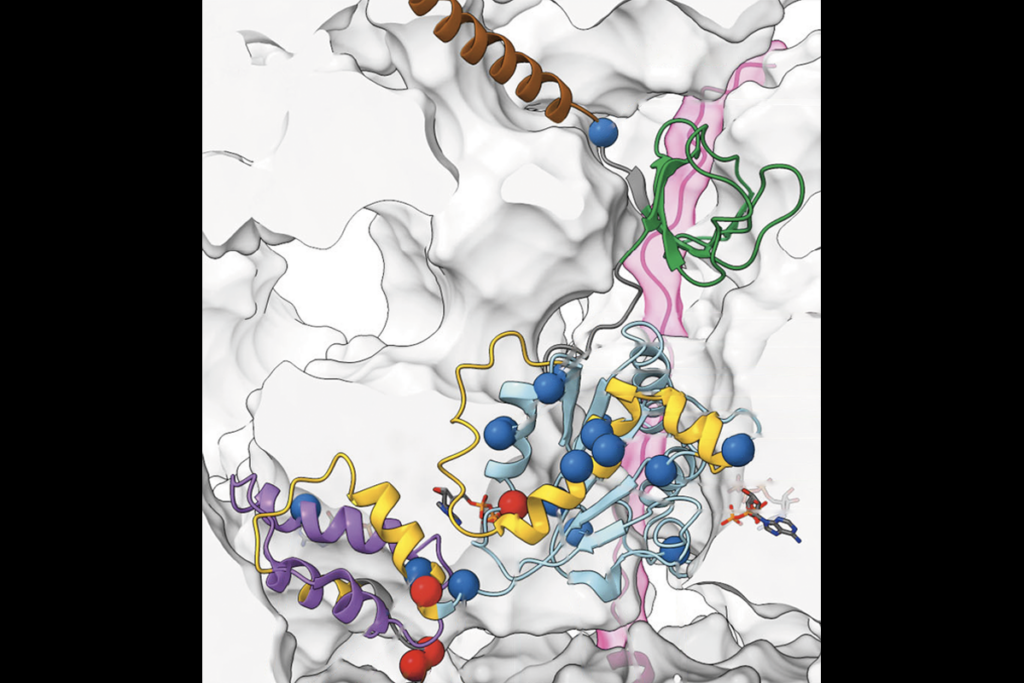
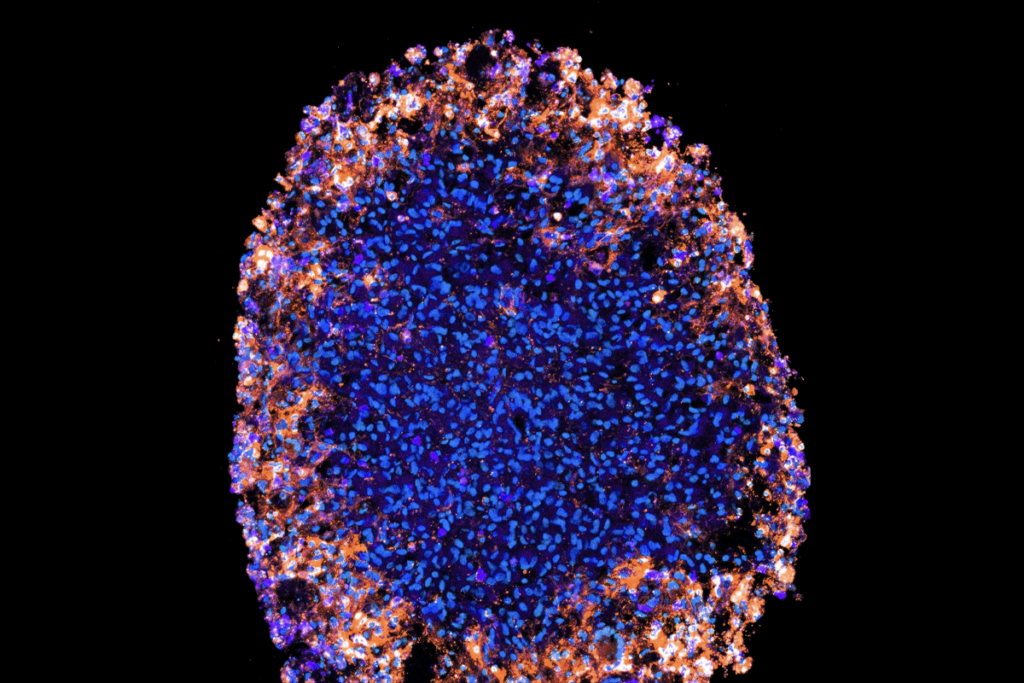
Long-range connections between the amygdala, which helps process emotions, and cortical regions that power cognition help us manage our behavior and navigate social interactions.
Now scientists have identified genes that direct developing neurons in the amygdala and its surrounding areas to forge these vital connections. “We have found a key to opening a part of this mysterious region,” says investigator Navjot Kaur, associate research scientist in neuroscience in Nenad Sestan’s lab at Yale University.
Whereas neuron types in the prefrontal cortex and other parts of the neocortex self-sort into clear layers, disparate cells mingle in the amygdala. This intermixing makes it difficult to separate one neuron’s type from its neighbor’s—and to isolate the mechanisms that allow these distinct cell populations to develop and connect.
“It’s like looking at a beautiful six-layer fruit cake with distinct fruits in each layer where you can see, taste and appreciate each layer, versus a finely chopped fruit salad,” Kaur says.
Amidst the cell mélange of the amygdala’s basolateral area, Kaur and her colleagues sought out which neurons form connections with the prefrontal cortex. Disruptions to those links are associated with several conditions, including autism, schizophrenia, anxiety and depression. The basolateral amygdala, as well as the claustrum and piriform cortex, all shrink in mice missing the transcription factors SOX4 and SOX11, which past work has associated with significant developmental defects in other brain areas. The findings appeared in a preprint the team posted 20 October on bioRxiv.
Knocking out those two genes also downregulates expression of another transcription factor, TFAP2D, the team found. In human, rhesus macaque, chicken and mouse brain samples from the basolateral amygdala complex and its neighbors, developing excitatory neurons express TFAP2D, implicating those cells in linking these regions to cortical ones.
“These sorts of advances are essential to understand … what may be altered in neurodevelopmental disorders or in adults with neuropsychiatric conditions,” says Shawn Sorrells, assistant professor of neuroscience at the University of Pittsburgh, who did not work on this study.
S
OX4 and SOX11 appear to regulate TFAP2D activity; for instance, SOX11 can bind to six putative enhancers of TFAP2D activity.Mice with only one functional copy of TFAP2D possess an intact basolateral amygdala, Kaur and her colleagues found, but those with no working copies do not. However, both model animals experience disrupted connections between the basolateral amygdala and the prefrontal cortex, resulting in increased conditioned fear responses when the animals learn to associate sounds with mild foot shocks. “This insight reveals how distinct anatomical variations can produce similar behavioral issues in adulthood,” as is the case in many neuropsychiatric conditions, Kaur says.
Although the excitatory neurons in the basolateral amygdala are among the earliest born neurons, their connections with the prefrontal cortex develop after birth and continue to mature into adolescence. “This long-protracted window of development makes these neurons and their circuits highly vulnerable to environmental influences,” which genetic predispositions may exacerbate, Kaur adds.
“If we can identify genetic predispositions [such as having only one functional copy of TFAP2D] early on, we might be able to intervene during this window to reduce the risk of developing neuropsychiatric disorders,” Kaur says.
To see how genetic and environmental factors might interact, the scientists say they want to investigate whether a lack of caregiving or an impoverished upbringing leads to greater disruptions of basolateral amygdala connections in animals with just one functional TFAP2D gene, and whether, by contrast, “an enriched environment reduces these connectivity deficits,” Kaur says.
The team also aims to identify TFAP2D’s target genes to clarify the molecular and cellular features of basolateral amygdala development, Kaur says. In addition to fear-related experiments, they say they would like to observe the social behavior of TFAP2D knockout mice to see the gene’s effects on other circuits related to the basolateral amygdala.
Additional work should also explore how disease-associated mutations might interact with the gene network involved and model the findings in human cerebral organoids or other species, Sorrells adds.
“Future research should aim to establish a detailed profile of neuroendocrine and motivational changes in TFAP2D knockout mice and explore the timing and effects of identified enhancers on amygdala-prefrontal connectivity in rodent and nonhuman primate models of amygdala development,” says Vincent Costa, associate professor of developmental and cognitive neuroscience at the Emory National Primate Research Center, who did not contribute to this study. “Applying similar methods to primates would help to verify the translational relevance of these findings.”