Mini-brains bare tortuous trek of cells in Timothy syndrome
A new technique for building a ‘brain in a dish’ reveals how neurons move to their proper places during fetal development.
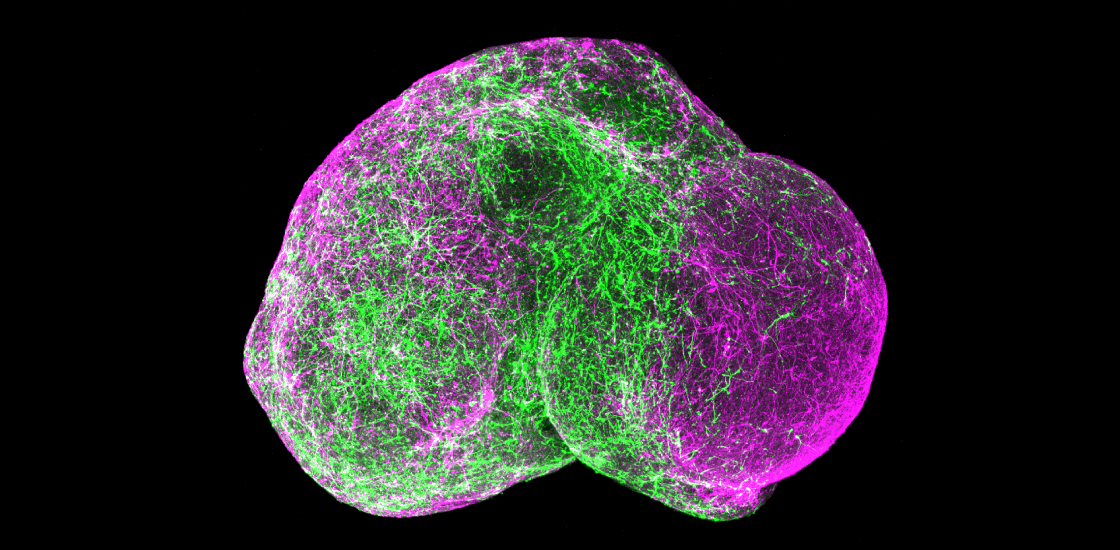
A new technique for building a ‘brain in dish’ reveals how neurons move to their proper places during fetal development — and how that process may go awry in people with a genetic condition linked to autism1.
The method is a twist on a technique used to grow neurons in 3-D clusters called spheroids. The clusters mirror the architecture of the brain’s outer rind, or cerebral cortex, better than cells grown in a single layer in a lab dish.
But a critical class of cells was missing from those spheroids: interneurons, which inhibit electrical activity in the brain. The balance between inhibitory and excitatory brain signaling is thought to play a role in autism.
In the new study, researchers used a cocktail of growth factors and other molecules to make spheroids that mimic the subpallium, a deep brain region where interneurons form. During fetal development, interneurons migrate from the subpallium into the cortex, where they mature and make connections with excitatory neurons.
To model this process, the researchers put a spheroid of subpallial cells and a spheroid of cortical cells together in a dish. Within two or three days, the two spheroids began to merge, with cells from the subpallial spheroid migrating into the cortical one.
The migrating cells move in a “very peculiar way,” says lead researcher Sergiu Pasca, assistant professor of psychiatry and behavioral sciences at Stanford University in California. Every few hours, a subpallial cell sends out a long process that moves around as though it’s searching for a target, and then the body of the cell ‘jumps’ to catch up. The findings appeared 4 May in Nature.
Short hops:
Pasca and his team also made spheroids containing neurons derived from the skin cells of people with Timothy syndrome, a rare genetic disorder associated with autism and epilepsy. The syndrome stems from a mutation that sends a calcium pump on neurons into overdrive.
Interneurons from people with Timothy syndrome jump more often than neurons from controls, the researchers found. But they move a shorter distance with each jump, making their migration less efficient.
It is not yet known whether the number or location of interneurons is altered in the brains of people with Timothy syndrome. Pasca and his team are using the method to look at interneuron migration in other conditions associated with autism, such as 22q11.2 deletion syndrome.
Separately, they are also measuring changes in gene expression as interneurons migrate, mature and form connections with excitatory cells. The genes involved in these processes could yield clues to autism and other neurodevelopmental conditions.
The team is also creating recipes for spheroids that mimic other regions of the developing brain, such as the striatum and the hippocampus. They could then put these spheroids together in various combinations. “It’s essentially a modular system,” Pasca says.
References:
- Birey F. et al. Nature 545, 54-59 (2017) PubMed
Recommended reading

Expediting clinical trials for profound autism: Q&A with Matthew State

Too much or too little brain synchrony may underlie autism subtypes
Explore more from The Transmitter

Mitochondrial ‘landscape’ shifts across human brain

