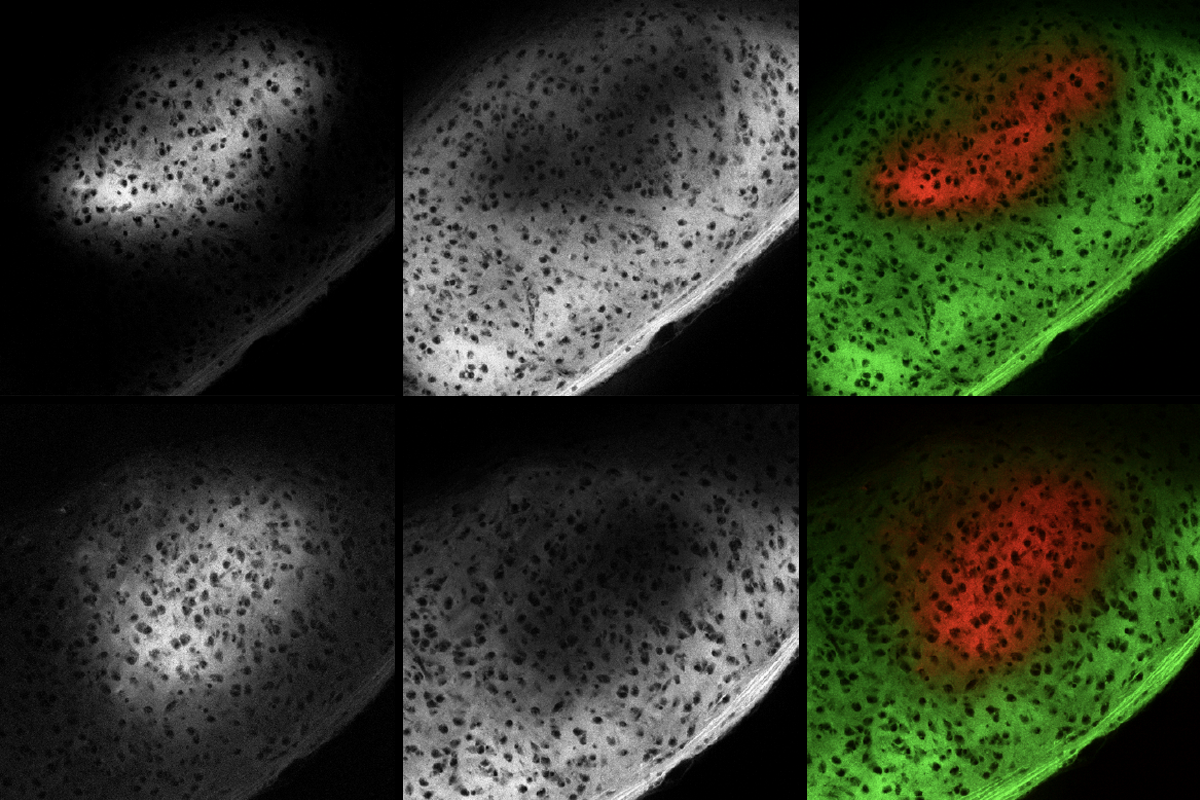
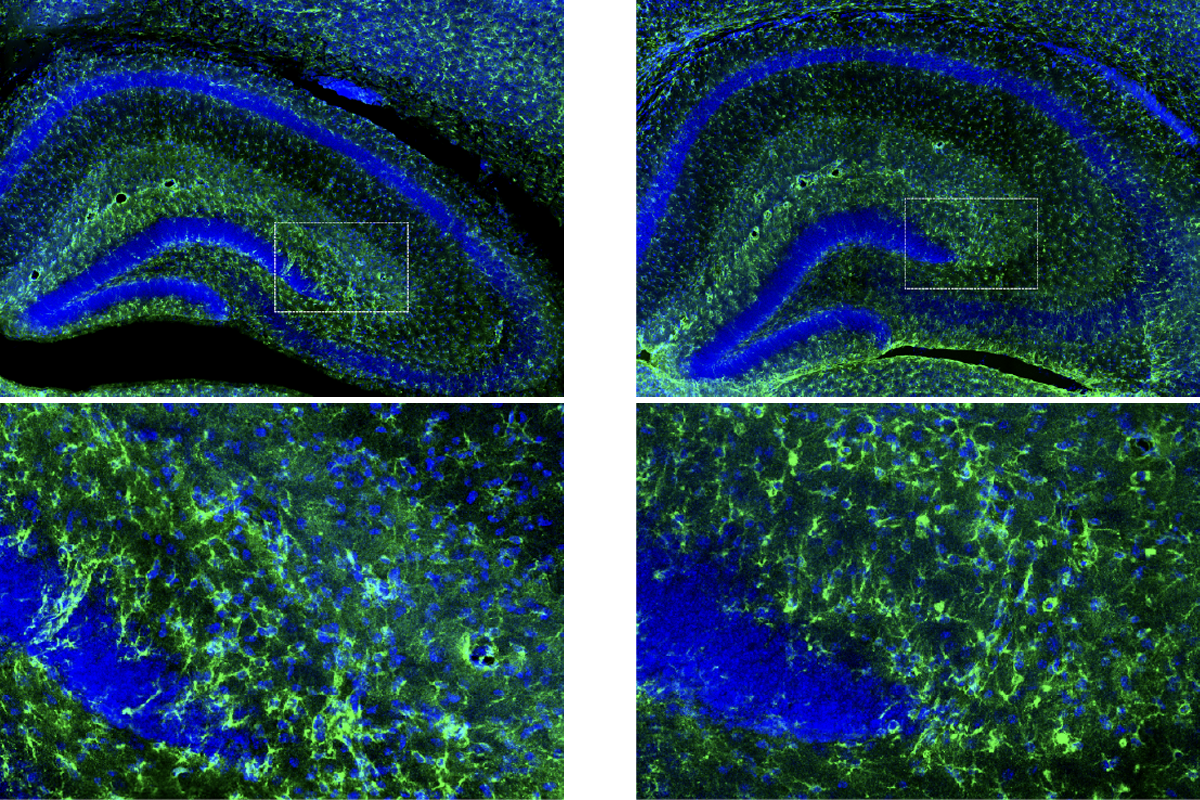
Microglia were once thought to have one job—as the brain’s resident garbage collectors. If neurons became damaged or diseased, microglia would spring into action, engulfing dead or infected cells and pumping up the local immune response. Between clean-up operations, scientists believed, they rested in a deep sleep.
In 2005, though, researchers got their first direct look at what microglia were doing in the brain, and they promptly tore up this cellular CV. The grainy live-cell imaging footage, published in Science, showed that the supposedly “resting” microglia were actually marauding around in the neocortex of adult mice, firing out processes and furtively feeling out the surrounding parenchyma.
“This, for me, was a game changer,” says Rosa Paolicelli, associate professor of biomedical sciences at the University of Lausanne, who was about to embark on a Ph.D. at the time. “People started to think about the physiological role of microglia. What do they do in the intact, healthy brain?”
The work kick-started two decades of research that has changed how the field classifies microglia, and led to new tools to help scientists define and scrutinize the cells’ functions in detail. Many studies have focused on “critical windows”—at the beginning and end of an animal’s life, Paolicelli says. During these time frames, microglia take on many side jobs: as sculptors of the developing brain, cultivators of new neural connections and fighters of neurodegeneration, for example.
Along with this rise in profile from garbage collector to cellular polymath has come controversy. “There are some players that are trying to bring forward some ideas that are a bit too simplistic or restrictive. And I feel it’s very dangerous not to stay open-minded regarding the implications of the findings, not to stay open-minded regarding the limitations of all these models,” says Marie-Ève Tremblay, professor of medical sciences at the University of Victoria, who studies microglial function in health and disease.
What microglia do during early development has proved particularly contentious. At least three mouse studies published this year have called into question the extent to which microglia snip away synapses to shape a budding brain.
These findings continue to refine the cells’ task list and work schedule, supporting the idea that their functions depend on timing and context, and opening new avenues for research, says Beth Stevens, associate professor of neurology at Harvard Medical School. “We need to really get mechanistic and really start to think about developing the tools and the ways to be able to manipulate the right pathway at the right time to ask specific questions,” she says.
S
oon after the 2005 Science paper came out, Stevens was a postdoctoral researcher in the late Ben Barres’ lab at Stanford University, studying developing visual circuits in newborn mice. Immune molecules called complement proteins appeared to have a role in shaping these circuits by “tagging” redundant synaptic connections for elimination, the team reported in 2007 in Cell. And microglia, a 2012 follow-up showed, devoured the tagged synaptic connections, helping to sculpt the brain.These results unmasked a whole new identity for these cells—like finding the Batcave under a microglial Wayne Manor. But recent work has drawn some of the cells’ superhero status into question. For instance, mice depleted of microglia starting 14 days after birth develop no discernible problems with visual performance, according to a July Nature Neuroscience study.
“We didn’t find any difference for any of the measurements we made across the different experimental approaches we used to examine the functional aspects of circuitry,” says the study’s lead investigator, Aaron McGee, professor of translational neurosciences at the University of Arizona. “Microglia perform immuno-surveillance; they do not sculpt brain circuits.”