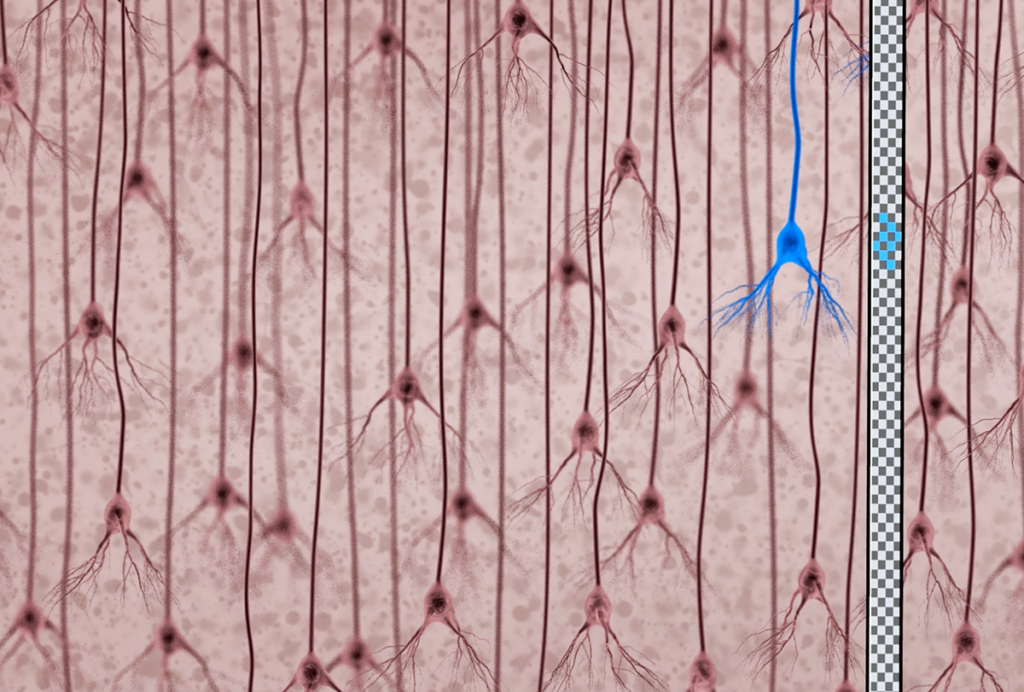
Erasable sensor of brain activity could reveal roots of social behavior
Researchers unveiled a reversible new technique for labeling active neurons in freely moving animals.
Researchers unveiled a reversible new technique for labeling active neurons in freely moving animals.
The method enables scientists to monitor the neural activity of a single animal over multiple trials and in response to a variety of stimuli and conditions.
“This allows you to mark an active ensemble of neurons, erase it, and then re-mark another ensemble,” says Fern Sha, a postdoctoral researcher in Eric Schreiter’s lab at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia.
Researchers presented the unpublished findings today at the 2019 Society for Neuroscience annual meeting in Chicago, Illinois.
The work builds on a technique known as CaMPARI, which Schreiter and his colleagues described in 2014. The technique relies on a fluorescent protein that responds to changing calcium levels in neurons.
Researchers engineer neurons to express this protein, which makes them glow green. When the neurons fire, calcium flows into the cell. Exposing the calcium-flooded neurons to violet light causes the fluorescent protein to switch from green to red.
In the previous version of the system, this color change is permanent. “It was basically a one-shot tool,” Sha says.
The new, reversible system, known as rsCaMPARI, relies on a different protein that also turns engineered neurons green. When researchers expose an active calcium-flooded neuron to blue light, the green fluorescence dims, turning the neuron dark. “The fluorescence turns off in active neurons,” Sha explains.
Scientists can reset the system by exposing the neuron to violet light, which causes the neuron to fluoresce bright green again. They can repeat this procedure for roughly 10 cycles, Sha says.
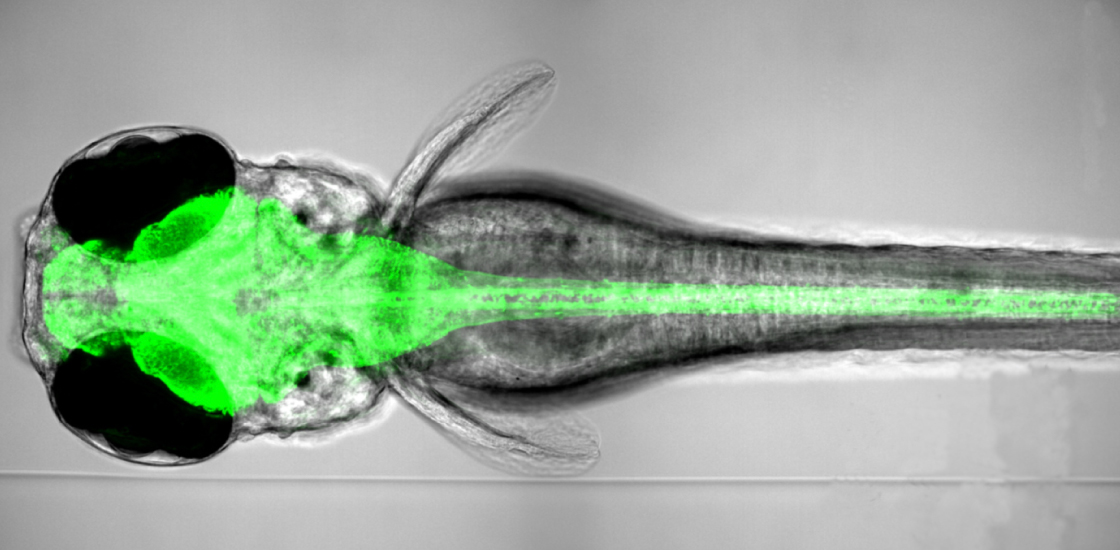
The team demonstrated the system in cultured rat neurons and freely swimming zebrafish.
On the fly:
In a separate presentation today, another team showcased the use of CaMPARI in freely moving fruit flies. Previous versions of the technique required single flies to be immobilized while their brains were illuminated by a laser.
“[That approach] limits the movement of the fly,” says Katie Edwards, a graduate student in Giovanni Bosco’s lab at Dartmouth College in Hanover, New Hampshire, who presented the work. “You can only look at so many behaviors that way and you can only do one fly at once.”
The team instead wanted to develop a method that was high-throughput and in which the flies could be freely moving, she says.
In the new method, the researchers put eight fruit flies in a single Petri dish and place a small violet-colored LED on top of each dish. The light shines on the flies as they move around the dish, turning the insects’ active neurons from green to red. The method enables scientists to study the neural circuits involved in complex fly behaviors, including social ones.
For more reports from the 2019 Society for Neuroscience annual meeting, please click here.
Recommended reading
Documenting decades of autism prevalence; and more

Expediting clinical trials for profound autism: Q&A with Matthew State
Explore more from The Transmitter
‘Perturb and record’ optogenetics probe aims precision spotlight at brain structures