A form of CRISPR editing can modify expression of oxytocin receptors in at least six — and possibly more than 80 — species of rodents, according to a new study. The tool, which enables cross-species comparisons of how the receptors shape social behaviors, could also be adapted for other genes, the researchers say.
Oxytocin is famous for its influence on social behaviors, such as the bonding that happens between mother and child in many animal species, or between partners in some. The molecule, or a similar form of it, is evolutionarily conserved, but species differ in their patterns of expression of oxytocin receptors throughout the brain.
Scientists can easily manipulate these receptors in mice, which have a vast array of genetic lines. But when it comes to studying social behaviors, other species can offer insights that lab-bred mice cannot, says lead researcher Larry Young, director of the Center for Translational Social Neuroscience at Emory University in Atlanta, Georgia. Prairie voles, for example, pair bond; spiny mice live communally.
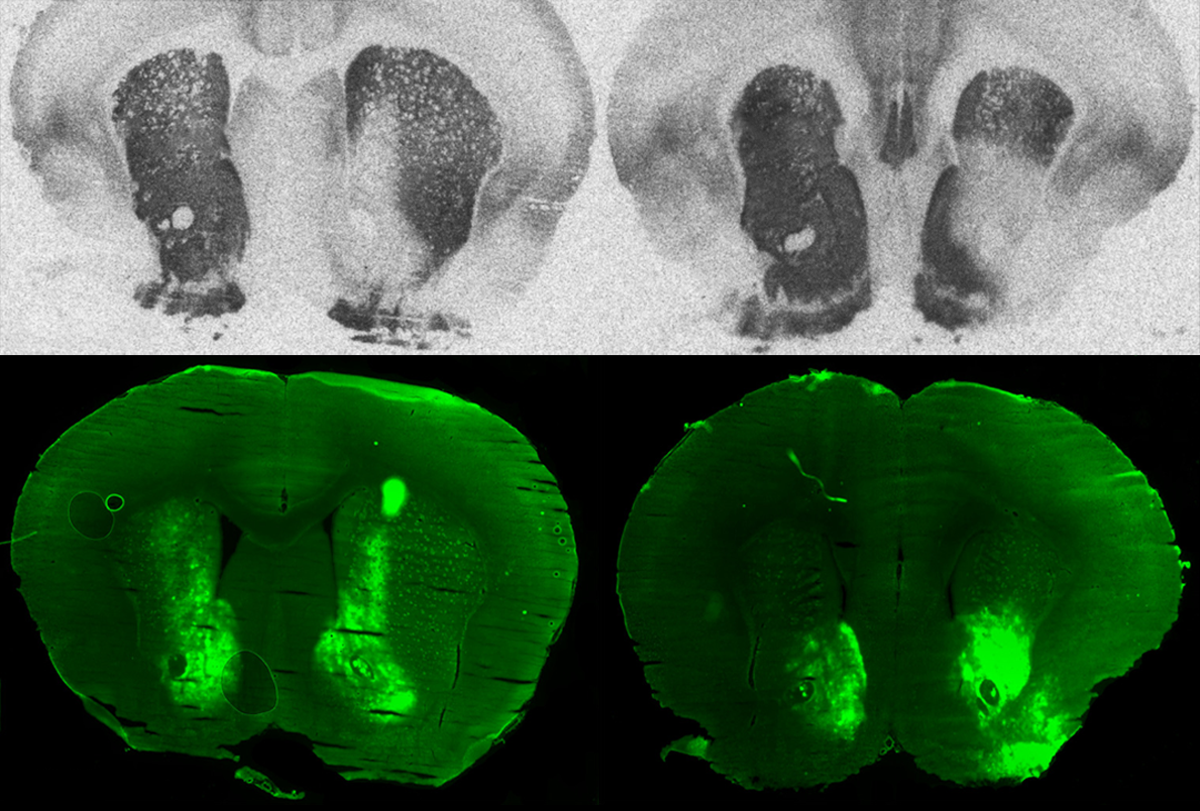
Because the oxytocin receptor gene differs across species, designing a new CRISPR guide RNA for each “was going to be a lot of work,” says study investigator Arjen Boender, who is a research associate in Young’s lab. So he compared the genetic sequences of four rodent species and found two conserved regions that might serve as cross-species targets. He and his colleagues designed new guide RNAs aimed at those regions, packaged them in viruses and sent the tools to rodent researchers across the United States.
One of the guides limited expression of the receptor for all six species tested — prairie vole, golden hamster, house mouse, California deer mouse, Norway rat and spiny mouse — the collaborators found when they injected the virus into a region of the brain known to typically be dense with the receptors. In prairie voles, it was not neurotoxic and did not affect expression of a vasopressin receptor, despite the close connection between the gene that encodes them and those that encode oxytocin receptors. The study was published last month in Science Advances.
The work represents a pendulum swing in the field after decades of focus on transgenic mice, says Robert Froemke, a professor in New York University’s Neuroscience Institute and otolaryngology department. It will, he says, “enable a lot of interesting questions to be asked in other species.”
Y
oung and his colleagues are studying the behavioral effects of using CRISPR to manipulate the expression of oxytocin receptors in prairie voles. Prairie voles engineered to lack functioning copies of these receptors still form pair bonds, a recent study revealed. But dampening their expression later in life, and in specific brain regions, may have a different effect, Young says.That kind of experiment, though standard in lab mice, could offer new insights into the neural underpinnings of social behaviors in prairie voles and other non-traditional rodent species, he says. That is something that Young and his colleagues say they hope to enable by making their tool broadly available.
“The benefit is that it’s off the shelf,” Young says — meaning that researchers need no experience designing CRISPR tools to use it. And as this type of tool reaches more labs, and more species are studied, he says, that makes the field as a whole stronger.
“It gives greater possibility to say, ‘What kinds of things are generalizable across species?’” Young says. “Which then makes it more likely to be generalizable to humans.”