Autism model reveals brain processes behind ‘super’ skills
Structural changes in the connections between neurons may underlie the enhanced learning and motor skills of a mouse model of autism.
Structural changes in the connections between neurons may underlie the enhanced learning and motor skills seen in mice with an extra copy of the autism-linked gene MeCP2. Blocking these changes with a drug blunts the animals’ performance.
The findings, presented yesterday at the 2015 Society for Neuroscience annual meeting in Chicago, point to neural mechanisms underlying the restricted interests and, in some cases, exceptional learning abilities seen in people with autism.
“This could lead to enhanced learning and enhanced performance in constrained behaviors, like in autistic savants,” says Ryan Ash, a graduate student in Stelios Smirnakis’ lab at Baylor College of Medicine in Houston. “Maybe they can’t iteratively refine those kinds of behaviors over time, so they get stuck in a behavior, which can be exceptional in certain cases but then impaired in others.”
People carrying an extra copy of MeCP2 often have autism. Mice with the same duplication have autism-like symptoms, such as avoiding social interactions with other mice.
“But they also have a super-learner phenotype,” Ash says. They perform better than controls do on a test of motor skill learning that involves balancing on a rotating rod. Typical mice fall off the rod as its speed increases, but mice with the duplication learn to coordinate their feet so that they can stay on about 30 seconds longer.
When mice learn a motor task, new synapses, connections between neurons, form in the brain1. The researchers suspected that the superior learning abilities of the mice carrying the extra MeCP2 might stem from alterations in the formation and stability of these neuronal links.
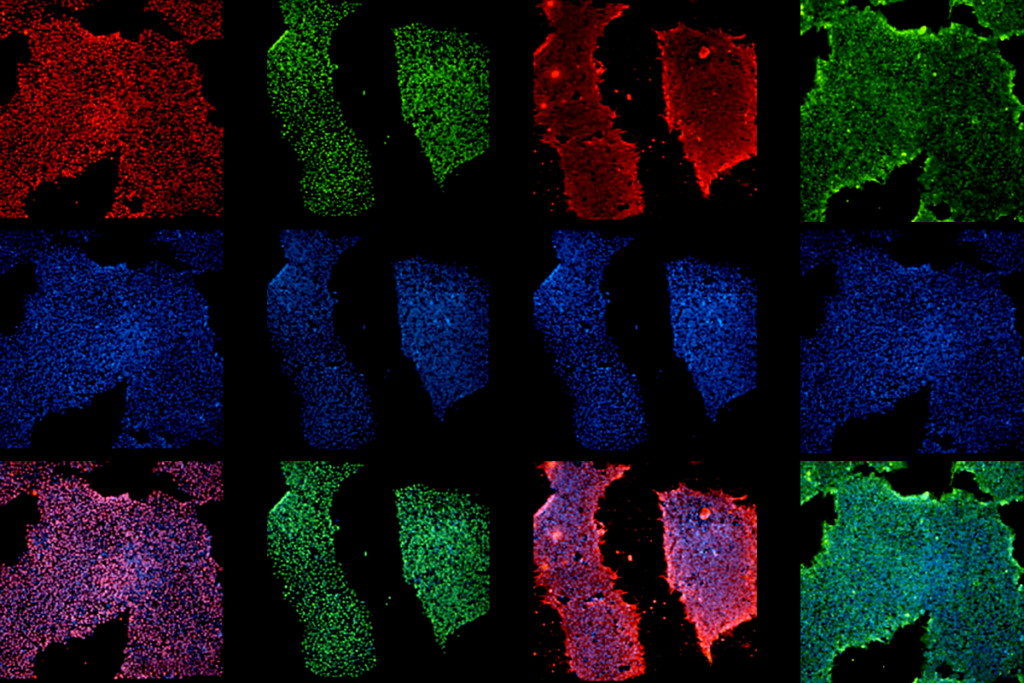
To test this hypothesis, the researchers used microscopy to image neurons in the brain that connect to the spinal cord and control movement. They took pictures of the same neurons before and after the mice practiced the rotating rod test for four days, and again after the animals had four days of rest.
Spine support:
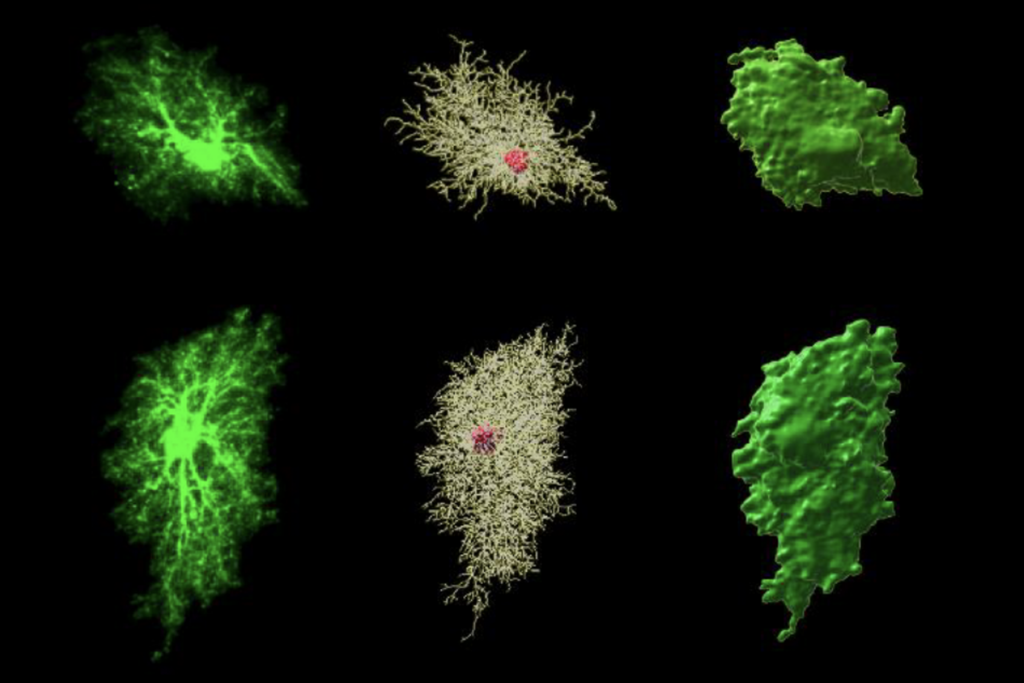
As expected, training spurred neurons in typical mice to form new signal-receiving projections, called dendritic spines. About half of these spines remained after four days of rest, suggesting the formation of stable memories. Mutant mice form more spines than controls do, and more of them stay put after the mice take a break.
The stable spines tend to cluster. Enhanced performance on the rod tracks with a greater number of clustered spines remaining after the rest period.
“We think this is important because spines that are near each other can drive the cell more strongly when they get activated at the same time,” Ash says.
Training stimulates greater activation of a signaling cascade called the RAS pathway in the mutant mice than it does in controls. Activation of this pathway is known to strengthen clustered spines2.
Blocking the activation of this pathway with an experimental drug called SL327 lowers the mutants’ performance on the rotating rod back to the normal range. And the spines in these animals also look more like those of typical mice.
The findings suggest that spine formation and stability underlie the enhanced learning abilities of the mutant mice. Both processes appear to depend on the activation of the RAS pathway.
The drug the researchers used lasts only for a few hours, so it is not likely to help people with autism, Ash says. But cholesterol-lowering drugs called statins block activation of the same pathway by a different mechanism. “Maybe you could do a more chronic treatment with a statin, but we haven’t tried that yet,” he says.
Other mouse models of autism show enhanced performance on the rotating rod test. These include mice with a duplication in chromosomal region 15q11-13 and with mutations in the CNTNAP2, NLGN3 and NRXN1 genes, Ash says.
Interestingly, mice that lack a copy of MeCP2 — the gene mutated in the autism-linked disorder Rett syndrome — have impaired performance on the same test, and show reduced spine stability. “I would hypothesize that all of these things are actually the opposite in the Rett mice,” Ash says.
For more reports from the 2015 Society for Neuroscience annual meeting, please click here.
References:
Recommended reading

Common and rare variants shape distinct genetic architecture of autism in African Americans

Profiles of neurodevelopmental conditions; and more
Explore more from The Transmitter
How artificial agents can help us understand social recognition
Methodological flaw may upend network mapping tool