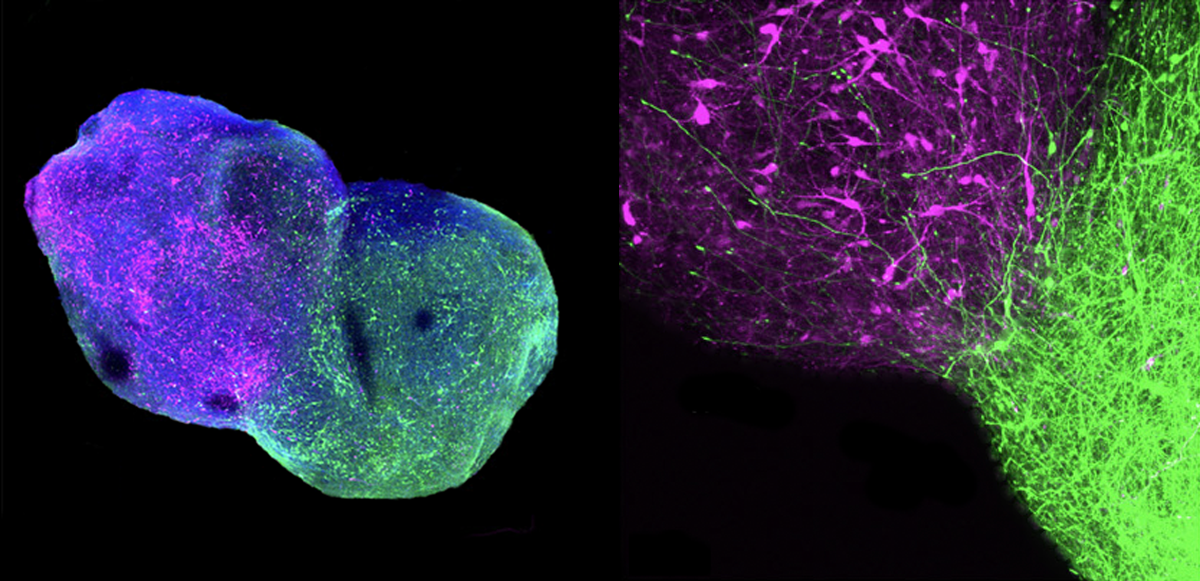
A few months ago, Sergiu Paşca, professor of psychiatry and behavioral sciences at Stanford University, shared his lab’s new work at the Gordon Research Conference on Thalamocortical Interactions. His talk concerned assembloids, lab-grown combinations of spherical organoids that mimic different parts of the nervous system.
Paşca showed a video depicting waves of calcium signals traveling along a line of organoids modeling sensory neurons; the dorsal root ganglia of the spinal cord; a subcortical structure called the thalamus; and, finally, the cerebral cortex.
In the audience, Audrey Brumback, assistant professor of neurology and pediatrics at the University of Texas at Austin, felt something move through her own subcortical structures as she watched the video: a visceral feeling of awe. “I just thought, ‘Holy crap, this is amazing,’” she recalls. “‘The future is now.’”
The work, described in a preprint posted on bioRxiv in March, is part of a series of recent studies from Paşca’s lab that highlight the potential of assembloids to help researchers understand brain development at the circuit level, and how these circuits go awry in autism and other neurodevelopmental conditions.
Autism, after all, involves differences in how various parts of the brain connect with each other, Brumback points out. “So to be able to model that in vitro is exactly what we need to be doing to be able to understand these network dysfunction disorders,” she says.
For example, a lack of synchrony between the cortex and the thalamus is known to be associated with autism and schizophrenia, whereas too much synchrony between the two regions is implicated in absence seizures in epilepsy. Using a two-part assembloid representing this pair of brain structures, Paşca and his team probed the roots of these alterations in a study published 16 October in Neuron.
The researchers tested the effects of disrupting CACNA1G, a gene that encodes a building block of a calcium channel that is prevalent in the thalamus. Thalamocortical assembloids with a gain-of-function variant in CACNA1G that is associated with seizures exhibit increased activity in the thalamus, they found. So do assembloids lacking CACNA1G function, which is linked to schizophrenia.
“That was a surprise,” Paşca says. “Both of them pretty much have the same phenotype, but it turned out the mechanisms are different, and the consequences on the cortex are different.”
P
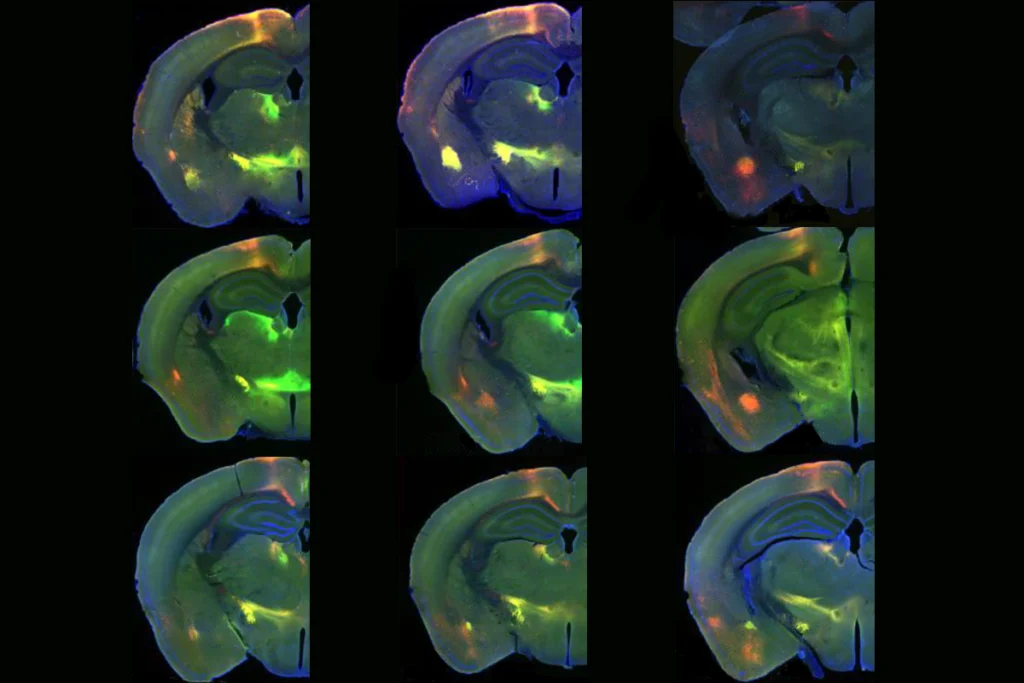
aşca and his team have previously investigated corticostriatal assembloids bearing genetic alterations related to autism. They have also constructed more complex assembloids, such as a three-part assembloid representing the cortex, spinal cord and skeletal muscles, in which signals from the cortical subunit produce contractions in the muscular one.Most recently, they completed a four-year quest to produce four-part assembloids, documenting the results in a pair of unpublished papers posted on bioRxiv. One features the linear sequence of organoids presented at the Gordon Research Conference, mimicking the pathway by which sensory information travels from the periphery to the brain. This assembloid could help researchers probe sensory alterations in autism, Paşca says.
The other assembloid quartet is composed of organoids that represent the cerebral cortex and three subcortical structures: the thalamus, striatum and midbrain. The four-leaf-clover arrangement models a loop circuit for the first time, tracing the flow of signals from the cortex to the striatum to the thalamus and back to the cortex. This pathway is important for understanding repetitive behaviors in autism.
Arranging the individual organoids in a loop “was very difficult,” Paşca says. “Every single time we would put them together in a circle, they would either fuse completely or completely detach,” he says. The team eventually came up with a set of 3D-printed wells resembling a cake-pop mold, which could temporarily hold the four organoids in the proper configuration until neuronal connections begin to form between them and stabilize the arrangement.
A
fter the assembloid is popped out of its mold and matures for another 100 days or so, the signals moving through the four organoids begin to synchronize, the researchers found. What’s more, loss of one copy of the gene ASH1L, which is implicated in autism and Tourette syndrome, increases this synchronous activity throughout the whole circuit.But it is the ability to build a loop circuit at all that is significant to other scientists who have studied assembloids, including Aparna Bhaduri, assistant professor of biological chemistry at the University of California, Los Angeles, who was not involved in the work. “I just think that is really cool.”