Expectations famously shape the experience of pain: People often report relief after taking a painkiller, even if they actually took an inert placebo.
A neuronal circuit that connects the rostral anterior cingulate cortex (rACC), the pons and the cerebellum may help translate that anticipation of pain relief into reality—at least in mice, according to a study published today in Nature.
The newly identified circuit activates as mice learn that a certain chamber in their cage provides relief from mild pain they experience in a different chamber. And turning on that circuit optogenetically can ease behaviors associated with pain, the study reports.
Those findings are surprising, because the cerebellum is best known for its role in movement and has not previously been linked to pain, says Ishmail Abdus-Saboor, associate professor of biological sciences at Columbia University, who was not involved in the study. “It changes how we think about the role of the cerebellum and cerebellar inputs for pain relief.”
Neuroimaging studies in people have previously identified rACC and some cerebellar activity in response to similar tasks, but the involvement of the cerebellum has been difficult to attribute to pain, says Grégory Scherrer, associate professor of cell biology and physiology at the University of North Carolina at Chapel Hill, who led the new work. “Because the cerebellum is a structure essential for movement and motor coordination, people have thought this was because, in the scanner, people had the intent to move.”
Discovery of this circuit in mice helps to replicate and advance those human findings, says Luana Colloca, professor of pain and translational symptom science at the University of Maryland School of Nursing, who was not involved in the work. “They added a new piece to the puzzle.”
S
cherrer and his team placed the mice in a chamber that had a hot floor—hot enough to cause the mice lick their paws and jump in pain, but not enough to cause lasting harm. They allowed the animals to move from there to a second chamber where the floor was cooler, so that they learned to associate that room with pain relief. Another group of mice—a control group—experienced two chambers at the cooler temperature, and thus never developed that association.When both rooms had hot floors, the conditioned mice preferred to spend time in the second chamber, whereas the control mice had no preference, the team found. The conditioned mice also showed fewer pain-associated behaviors after entering the second chamber than did the control mice, suggesting that their training led them to expect pain relief from that room and it somehow still soothed them, akin to a placebo effect seen in some people, Scherrer says. The effect lasted at least a week after the training took place.
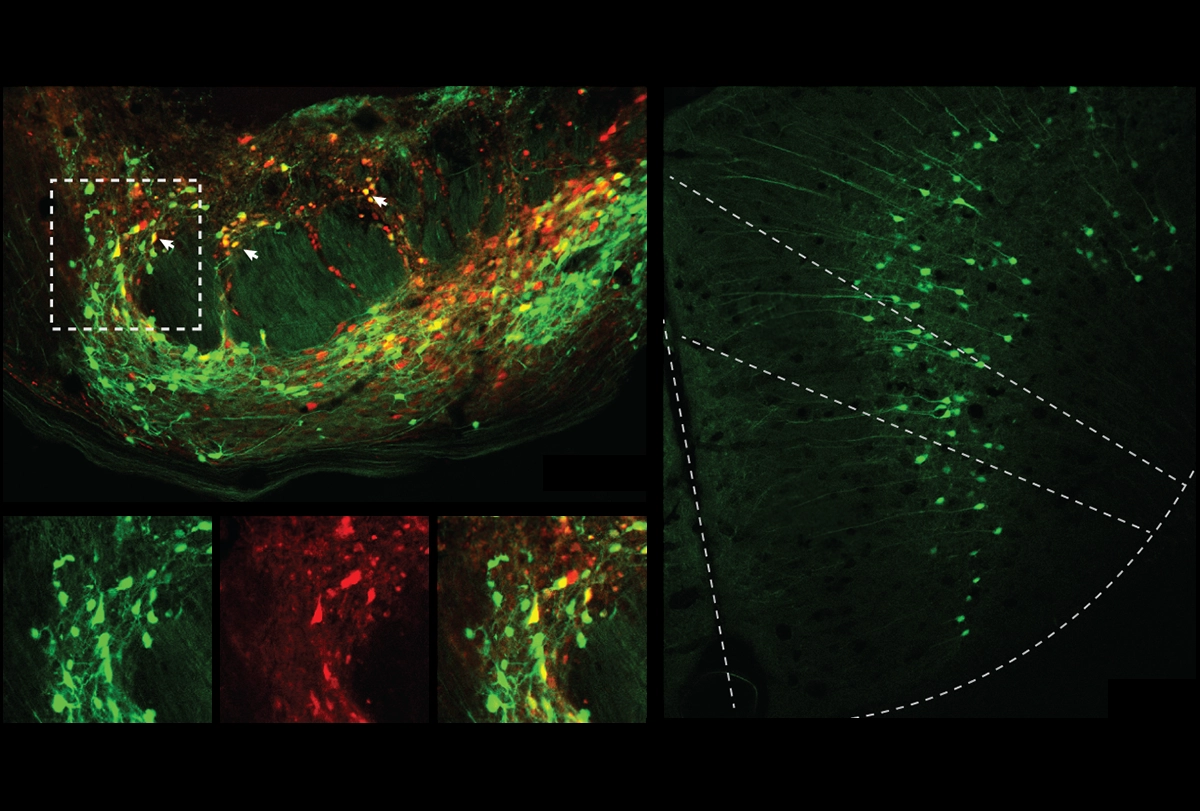
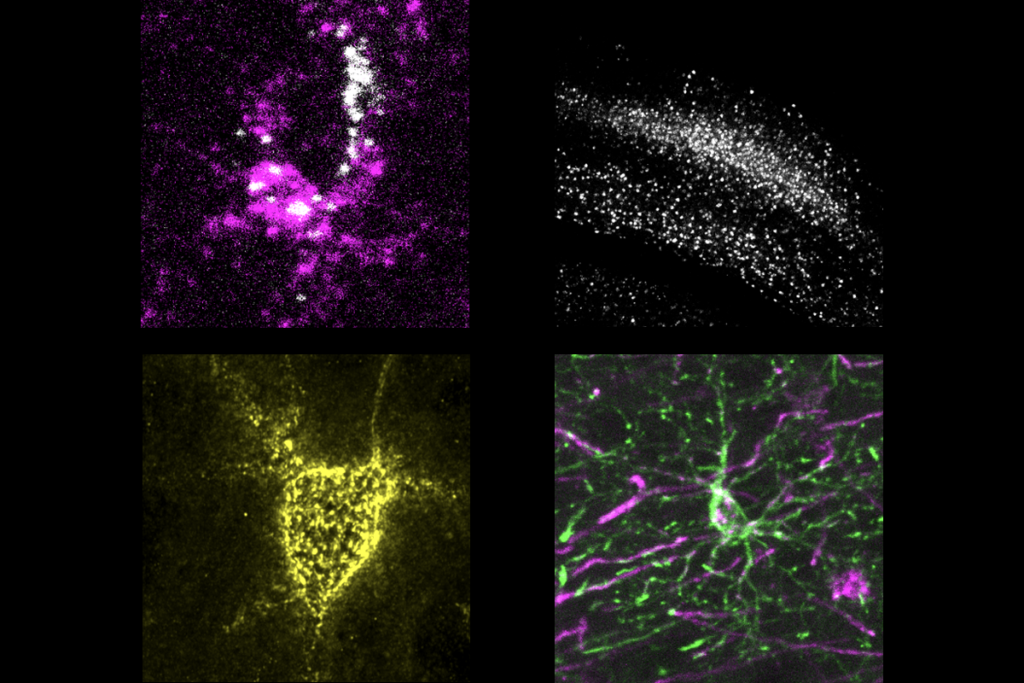
Upon crossing the threshold to that second chamber, neurons in the animals’ rACC that project to the pontine nucleus became active, according to measures of FOS protein levels, the team found. The rACC neurons ramp up in activity as mice learn to associate the second chamber with a decrease in pain, the team found using calcium imaging. “Other cortical neurons don’t do that,” Scherrer says.
Neurons in the pontine nucleus innervate the cerebellum. Inhibiting the rACC-pons-cerebellum circuit via optogenetics prevented mice from taking longer to show pain-related behaviors in the second chamber when both floors were hot; and activating the circuit in untrained animals postponed paw-licking but not other pain-linked behaviors.
That response comes down to opioid receptors, the team found. The pons neurons express genes encoding both delta- and mu-opioid receptors. Those cells project to cells that converge onto Purkinje cells in the cerebellum, which also respond to the seeming expectation of reduced pain.
R
esearchers have known that top-down modulation of pain taps into the opioid system: Administering naloxone, an opioid-receptor antagonist, to people can blunt the placebo effect, for example. The identification of this circuit may point to why, Abdus-Saboor says. Conditioned mice also failed to show a placebo-like effect when researchers gave them naloxone before making both floors hot, the new study shows.“It connects the dots with some indications that the field already had, but really provides a molecular-circuit-level analysis,” Abdus-Saboor adds.
An animal model of this form of pain relief has limitations, however, says Lauren Atlas, senior investigator at the National Center for Complementary and Integrative Health, who was not involved in the new work. For one, there is debate around whether the placebo effect relies upon the kind of conditioning that can be modeled in animals—or if it relies upon expectation, which is based on verbal instructions.
“The model used here really can only account for expectations that come about through learning,” which is one form of the placebo effect studied in people, she says. But other human studies have revealed that when a researcher tells a person that they are going to take a new drug that will increase their pain after being conditioned to expect a decreased pain response to the treatment for multiple days, that verbal cue has a stronger influence on a person’s pain perception than a conditioned response does.
The work also raises questions about how researchers think about the cerebellum, says Theanne Griffith, assistant professor of physiology and membrane biology at the University of California, Davis, who was not involved in the work. Although there has been a greater appreciation for its influence beyond movement and motor control, it is difficult to say that movement is not a factor, Griffith says. Inhibiting the circuit, for example, did have a slight, albeit statistically insignificant, effect on the animals’ ability to walk across a spinning rod in the study, she says. “Are we trying to make this distinction between cognition and movement that our brain isn’t making?”
Scherrer agrees that the need to form expectations and, based on predictions, plan future movements may help explain the cerebellum’s involvement. “This aspect of pain that’s about, ‘What can I do to make sure I have as little pain as possible in the future?’ is, I think, a very important aspect of pain experience that just hasn’t been studied much so far.” He says he and his colleagues plan to continue to investigate the outputs of the cerebellum and how they shape pain perception.
Further study of this circuit can help broaden the field’s understanding of how the brain processes pain, Griffith says. “It reinforces this idea that pain is a very complicated phenomenon: that it’s not really restricted to any discrete region in the brain.”