Immune cell interlopers breach—and repair—brain barrier in mice
The choroid plexus, the protective network of blood vessels and epithelial cells that line the brain’s ventricles, recruits neutrophils and macrophages during inflammation, a new study shows.
For decades, researchers have considered the brain “immune privileged”—protected from the vagaries of the body’s immune system. But building evidence suggests that the brain may be more immunologically active than previously thought, well beyond its own limited immune response.
The choroid plexus in particular—the network of blood vessels and cerebrospinal-fluid (CSF)-producing epithelial cells that line the organ’s ventricles—actively recruits immune cells from both the periphery and the CSF, according to a new study in mice.
The epithelial layer of the choroid plexus shields the rest of the brain from toxic substances, pathogens and other molecules that circulate in the blood. Dysfunction and neuroinflammation in the choroid plexus is associated with aging and many neurological conditions, such as amyotrophic lateral sclerosis and Alzheimer’s disease. Even in the absence of inflammation, the choroid plexus harbors immune cells, some of which reside in the space between the vessels and the epithelial layer, and some on the epithelial surface. During an immune response, it also contains recruited cells, such as macrophages and other leukocytes, and pro-inflammatory signals, previous research has shown.
But those findings offered only a snapshot of the cells’ locations, says Maria Lehtinen, professor of pathology at Harvard Medical School, who led the new work. “Just because [the cell] is in the tissue doesn’t mean it’s necessarily crossing or has gone in the direction that you anticipate that it would be going in.”
How the choroid plexus gatekeeps immune cells remains a big question in the field, says Michal Schwartz, a neuroimmunologist at the Weizmann Institute of Science, who was not involved with the new work.
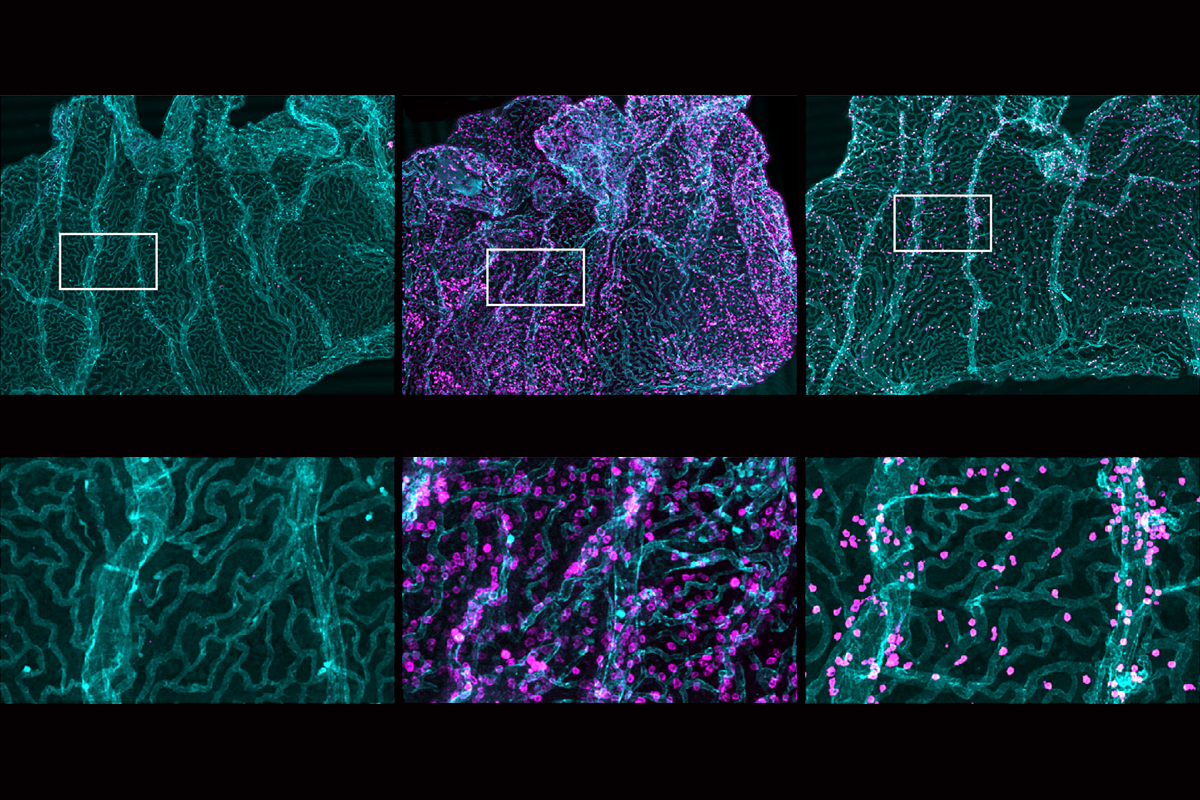
During inflammation, it turns out, the choroid plexus lining transiently breaks down and permits an influx of immune cells into the brain, the new study revealed using live imaging. And some of the epithelial cells within the choroid plexus activate recruited immune cells that help repair the damaged barrier.
The live imaging shows that the barrier is not static throughout the inflammation process, says Anna Molofsky, associate professor of psychiatry at the University of California, San Francisco, who was not involved in the work. “It opens people’s minds to the possibility that the barrier is dynamic, and that’s critical going forward.”
T
o get a real-time look, Lehtinen and her colleagues mounted a microscope over a window embedded in the skull of a mouse and measured the cellular activity across the choroid plexus through two-photon calcium imaging, a technique they had tested in previous studies of embryonic and awake adult mice. They then injected lipopolysaccharide (LPS), a component of bacteria, into the animals’ CSF-filled ventricles to mimic the inflammation associated with bacterial meningitis.Immune cells from the periphery flooded into the choroid plexus after the injection, the team observed. And immune cells—first neutrophils and later macrophages—breached the epithelial barrier and poured into the CSF, the team found, replicating previous findings. Movement also occurred bidirectionally: Immune cells flowed not only from the periphery, but also from CSF traveling toward the barrier.
That was unexpected, because the choroid plexus was thought to regulate only one-way passage from the blood to the brain, says Ryann Fame, assistant professor of neurosurgery at Stanford University, who was not involved in the new study. The findings highlight the additional ability of the choroid plexus to attract immune cells from the CSF and raises new questions about how the barrier can sense, respond to and regulate inflammation in the central nervous system, Fame adds.
Macrophages that reside in the choroid plexus help drive this influx, further experiments suggest. These cells’ level of inflammatory molecules peaked 24 hours after injection, according to single-cell RNA sequencing, hinting at their role in recruiting other immune cells. Similarly, some of the tissue’s epithelial cells transiently increased levels of cytokines, which facilitate macrophage survival and differentiation; adhesion molecules, which enable immune cells to attach like Velcro; and remodeling enzymes, which can change the extracellular environment to allow immune cell infiltration, the team found.
The barrier began to break down 24 hours after injection, evidenced by a decrease in tight junction proteins such as OCLN and CLDN2. But once the immune cells arrived, they set to work on repair: Five days after the insult, levels of critical tight junction proteins had returned to normal, and the barrier had healed. The barrier recovered much more slowly, however, when the team blocked the recruitment of macrophages with neutralizing antibodies.
“These inflamed epithelial cells orchestrated this whole stepwise program to help resolve and tackle this whole inflammatory state,” Lehtinen says. She and her colleagues published their findings in Cell in September.
T
he findings replicate and help explain some of what Lehtinen and her colleagues saw in the brains of people with bacterial meningitis, an infection of one of the brain membranes: Immune cells, including macrophages, seemed to leak into the ventricles, the team found.Still, the mouse models do not perfectly replicate human illness, Molofsky says. LPS is a “clean” way to study inflammation because it generates a robust and well-defined immune response, but moving forward, the field should look at more complex models, she says. “The main direction for the future is to begin to study these types of things in the context of more physiologic models of disease.”
Lehtinen says she is excited by the combination of live imaging with transcriptomics to study other barriers as well. “We’re still just looking at a very small amount of real estate in the brain. The brain has many different barriers—choroid plexus is one of them” she says. “[The approach] gives us the opportunity to start dreaming about these bigger questions and next steps.”
With these advances, the future may hold more investigations into the under-studied choroid plexus, Schwartz says. “The field is blooming.”
Recommended reading
‘Unprecedented’ dorsal root ganglion atlas captures 22 types of human sensory neurons
Psychedelics research in rodents has a behavior problem
Explore more from The Transmitter

Not playing around: Why neuroscience needs toy models

