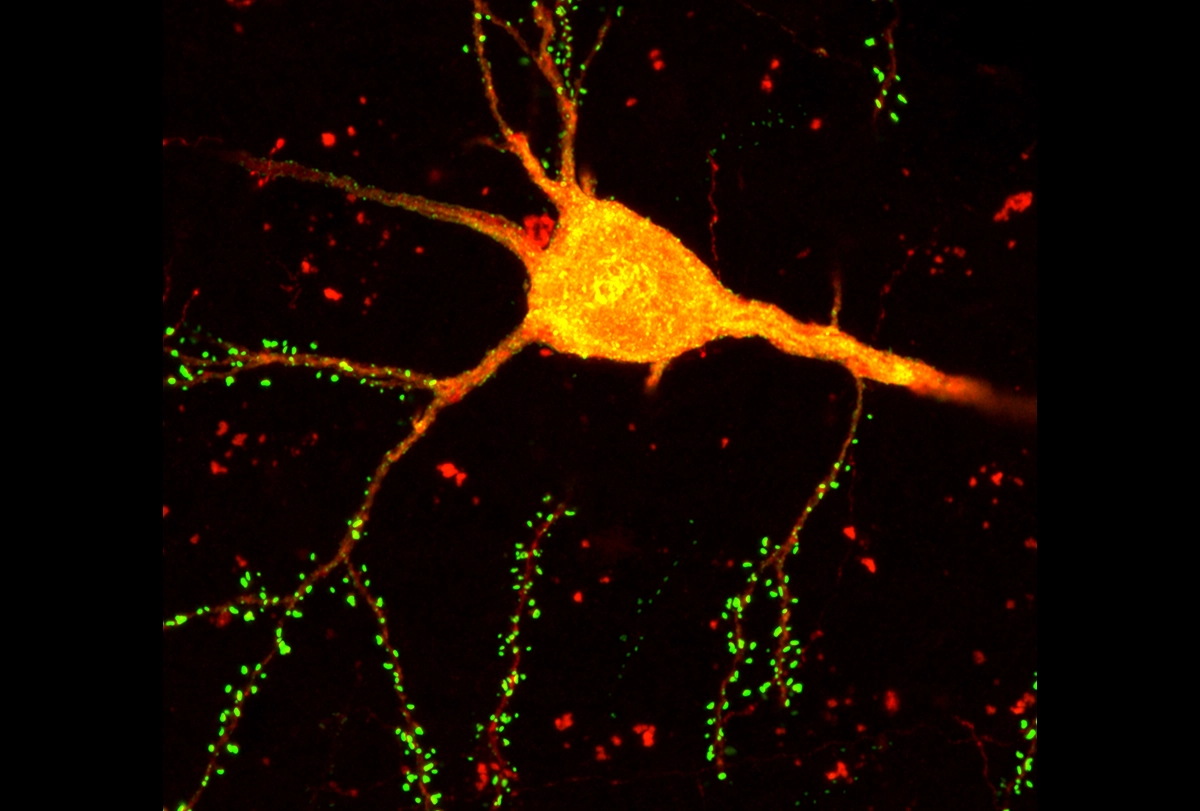
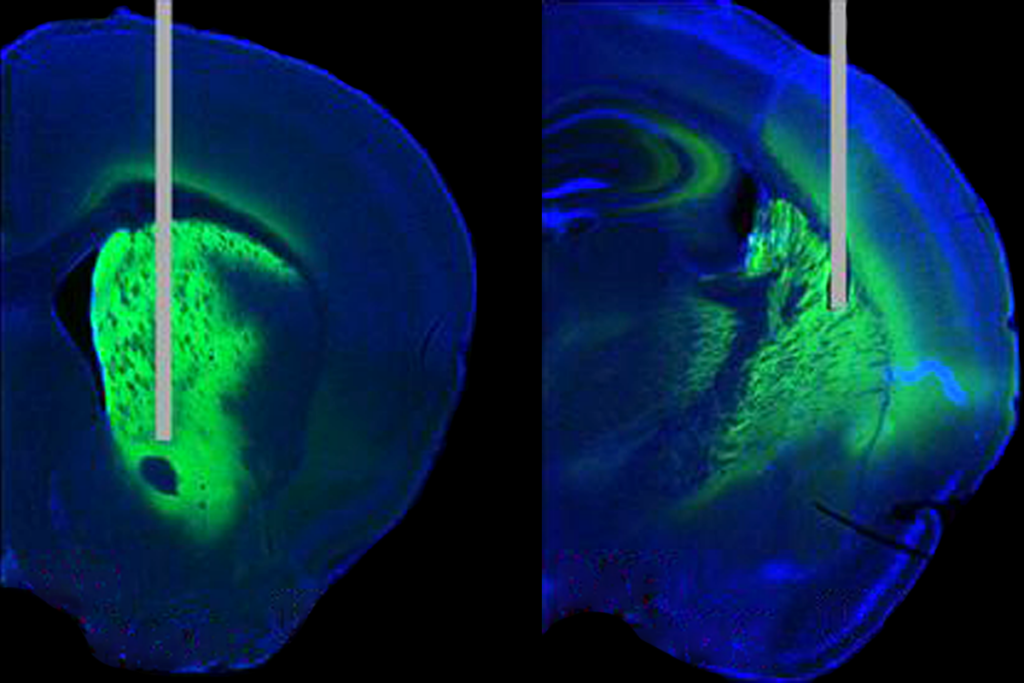
A new technique simultaneously identifies neuron types and charts their connections in the mouse brain. The tool, which combines neuronal tracing with single-cell sequencing, could help reveal how a cell’s identity contributes to cortical function, the researchers say.
The method—known as START, or “single transcriptome assisted rabies tracing”—organizes cells into groups based on their gene expression and traces their connections. If a group shows distinct connectivity patterns, it likely represents a distinct subtype, the study suggests. By applying the tool to the mouse visual cortex, the team pinpointed the connections among several subgroups of inhibitory neurons and discovered interactions that may be conserved in people.
“This really represents not only a technical breakthrough but offers a new framework for thinking about what [determines] a cell type,” says Xin Jin, associate professor of neuroscience at the Scripps Research Institute, who was not involved in the work.
START’s findings can be used to generate hypotheses about how cortical circuits work, says study investigator Edward Callaway, professor of systems biology at the Salk Institute for Biological Studies. “To understand how any complex system works, you need a parts list and a wiring diagram showing how those parts work together,” he says.
Past studies led by Callaway and other researchers, on behalf of the Brain Initiative Cell Census Network, produced that parts list. Using single-cell transcriptomics combined with other techniques, the consortium identified about 5,000 neuronal subtypes in the mouse brain. Now Callaway and his colleagues are working on the wiring diagram for those individual cell types.
In this case, the group combined transcriptomics with rabies tracing—a method also developed by Callaway’s team. By infecting neurons with a modified rabies virus that can jump only between synaptically connected cells, the researchers can track the immediate connections of select neurons.
They used the technique to trace inhibitory neurons, because their short-range connections can be detected within a single 400 cubic micrometer tissue sample. What’s more, inhibitory neurons are incredibly diverse, says study investigator Maribel Patiño, a graduate student at the University of California, San Diego. That individuality, even between neurons that belong to the same cell type, makes it tricky to match inhibitory neurons to certain brain functions, she adds.
T
he team traced the inhibitory neurons’ interactions with excitatory cells in a mouse’s visual cortex and then sequenced the nuclei from more than 35,000 neurons. They identified about 50 inhibitory neuron subtypes based on similarities in their gene expression.Patiño and her colleagues validated the technique by tracing previously described circuits in the visual cortex, such as known interactions within cortical layer five. But when they sorted the cells into transcriptomic subtypes, they uncovered previously unknown connections.
For instance, vasoactive intestinal peptide (VIP)-expressing neurons—previously known to inhibit other inhibitory neurons—comprise two groups that either seek out or avoid connections with excitatory cells. The findings hint that studies of VIP neurons may have been hindered by mixing multiple cell types with opposite functions, Callaway says.
And one type of layer six excitatory neuron—known to project to the thalamus to regulate the slow wave rhythms important for sleep and memory formation—received the majority of its inputs from a rare subtype of somatostatin-expressing neuron called Chodl cells, the study also found. Although Chodl cells make up just 1 percent of somatostatin neurons, they form more than 20 percent of connections to these neurons.
That preferential interaction likely represents a circuit motif that is conserved across species, Callaway says. In fact, his team’s next steps are to see whether the visual circuits are repeated in other cortical regions of the mouse brain, and in human tissue donated from people after surgery for brain tumors or epilepsy, he says.
The findings were published on 30 September in Neuron, and the sequencing data is publicly available on GEO.
T
he approach helps resolve “a major area of contention” of assigning cell type identity based on transcriptional differences, says Arnold Kriegstein, professor of neurology at the University of California, San Francisco, who was not involved in the work.Previously, researchers have been unsure whether different patterns of gene expression reflect differences in neuronal identity, or just fluctuations in cellular pathways. But observing whether the cells have distinct patterns of connectivity makes it possible to distinguish cell types from cell states, Kriegstein says.
And combining START with other methods could add an extra dimension to the data, says Martin Munz, assistant professor of physiology at the University of Alberta, who was not involved in the work. Incorporating spatial transcriptomics, for instance, could tell us the parts, how they interact and their location in the brain, he says.
Even with all that information, researchers can make only informed guesses at how circuits might function. But the ongoing development of enhancers targeting specific cell types could help researchers match circuitry to behavior, Patiño says. “It’s a great time for this [tool] to be coming out.”