About 10 years ago, whenever Takeshi Imai presented on his group’s tissue-clearing technique at scientific conferences, audience members asked the same question over and over: When will this work on live tissues?
“That was a long-standing dream in this field, and at that time, I always answered that that would be impossible,” says Imai, professor in the graduate school of medical sciences at Kyushu University.
Tissues are opaque because the substances in them scatter light to varying degrees. Tissue clearing aligns these different refractive indices by removing lipids or adding in chemicals. These processes make it easier to visualize cells marked with a fluorescent tag, but both are toxic to living cells, Imai says. And even nontoxic clearing agents have to be used at such high concentrations that they become toxic in practice. So, tissue clearing has only worked for studying cell anatomy in fixed tissue.
But now, Imai’s team and an independent group led by Ed Boyden have each identified refractive-index-altering compounds that work in living brains, without compromising cellular health and function. As a result, they can be used in conjunction with functional imaging. Both groups posted their methods as preprints to bioRxiv last month.
The new techniques do not produce completely transparent brains, as the methods for fixed tissue do, but instead increase the signal-to-noise ratio for any added fluorescent tags and the depth of cells visible using one- and two-photon imaging. Previous efforts to improve imaging resolution focused on the microscopes and not the tissue, says Boyden, professor of neurotechnology at the Massachusetts Institute of Technology.
“We were wondering, ‘What’s the most opposite thing we could do?’ And we realized that, to our knowledge, nobody had made the living brain itself more transparent,” Boyden says. “And so we thought we’d give it a go.”
I
n both methods, the researchers add a compound with a high refractive index to artificial cerebrospinal fluid (aCSF) and wash the mixture across the exposed brains of anesthetized mice. This raises the refractive index of the brain tissue, which in turn reduces light scattering and increases the visibility of cells deeper in the cortex.Tweaking the refractive index of tissue by adding compounds with different refractive indices is an old idea, but “to clear something in vivo and still have meaningful physiology is new,” says Hans-Ulrich Dodt, professor at the Vienna University of Technology, who wasn’t involved in either study but co-developed one of the first tissue-clearing techniques.
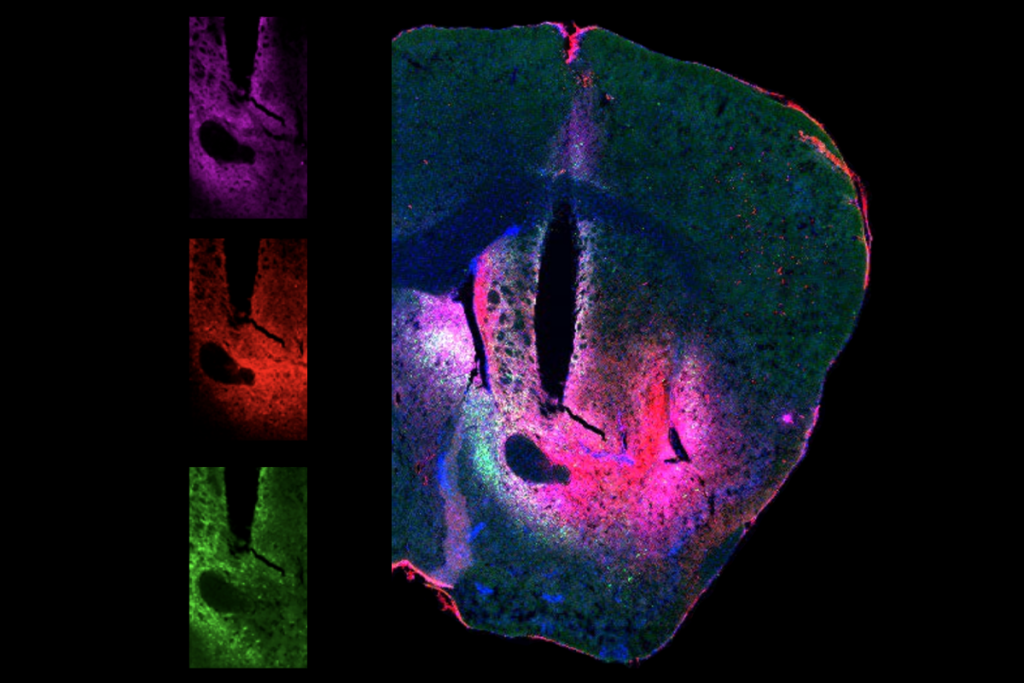
Imai’s group conducted a year-long screening process of compounds with a high molecular weight and a refractive index around 1.37 at a solute concentration in a healthy range for cells, Imai says. They settled on a solution containing bovine serum albumin, calcium and magnesium. Adding the formula, dubbed SeeDB-Live, to aCSF essentially makes a one-photon microscope as powerful as a two-photon microscope, Imai says.
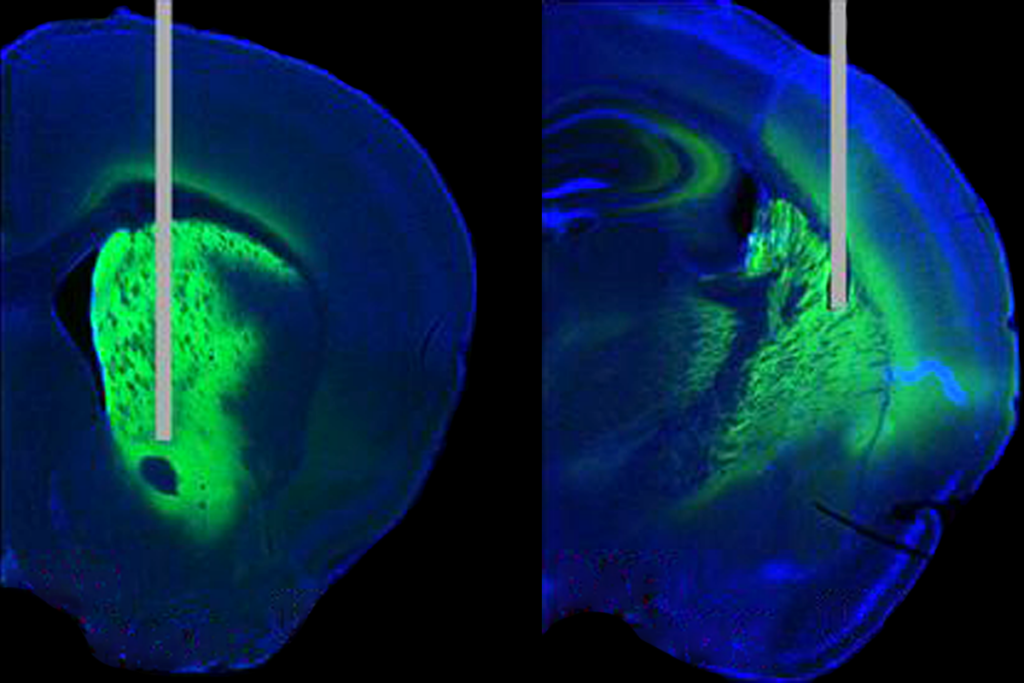
Boyden’s group also paid attention to osmolarity and molecular weight, but wanted to find an “off-the-shelf chemical,” Boyden says, because “we always want our techniques to be widely usable immediately.” The group identified the complex sugar dextran, which enhanced the brightness and increased the number of visible cells at a depth of up to 550 micrometers for two-photon calcium imaging.
This improvement will not be immediately helpful for researchers who study subcortical structures, such as Dayu Lin, professor of psychiatry, neuroscience and physiology at New York University. “It is a very cool, clever and simple technique,” Lin told The Transmitter in an email. “However, it will not make a big difference to our structures of interest,” including the amygdala and hypothalamus, “which typically sit more than 4 millimeters below the brain surface, much deeper than this method can reach (around 0.5 millimeters).”
But those deeper structures might become accessible if the clearing techniques are paired with a three-photon or other more powerful microscope, says Francesco Saverio Pavone, professor of physics at the University of Florence, who was not involved in either work. “That would be extremely cool.”
Imai’s clearing approach did not harm the health of the tissue, based on the firing activity of control versus cleared brain slices—a comparison that was “really tricky,” Imai says, because clearing the tissue makes it “very difficult to find” cells for patch clamping to assess that. And the function of neurons in the visual cortex remained intact, according to tests of the cell’s responses to visual stimuli through whole-brain calcium imaging.
Boyden also tested cell health, by measuring the orientation tuning of neurons in the visual cortex in awake mice. “We picked that because we thought it’d be a pretty demanding validation,” Boyden says. “Lots of stuff has to all go correctly for that to work.” There was no significant difference between control mice and mice with cleared brain tissue, the team reported.
Dodt says he would like to see even more validation that the normal functioning of the brain tissue is not affected by clearing before the technique is used in new studies. Boyden agrees: “Even with mature tools, I think people should be careful and do good controls. I think that that applies to all technologies, but definitely for a new technology.”
B
oth preprints followed an established tissue-clearing strategy, but a highly publicized paper published last month in Science—which used the dye from Doritos tortilla chips—took an entirely new approach, says Hiroki Ueda, team leader at the RIKEN Center for Biosystems Dynamics Research, who was not involved in the study.That team screened for compounds based on the frequencies of light they absorb, rather than their refractive indices. It’s “counterintuitive,” says study investigator Zihao Ou, assistant professor of physics and materials science at the University of Texas at Dallas, but absorbing light at a particular frequency reduces scattering and increases transparency at other frequencies. Tissue clearing has generally relied on the same “cocktail of chemicals,” and disregarded those that absorb light, Ou says, “but now we are providing more ingredients, so potentially we can provide easier and more effective options.”
Ou and his colleagues identified tartrazine, the yellow food dye in Doritos, as a potential clearing agent based on its absorption profile. When they applied it to the belly of a live mouse, the tartrazine cleared the skin and made the internal organs visible.
Those results are not particularly impressive or novel, Dodt says, but the approach is: Screening for substances based on their chemical and physical properties, such as absorption, is more rigorous than the typical trial-and-error used in the field—and he says he hopes the paper will attract more material chemists to work on the problem.
Ueda says he is curious to see if this technique will work in live brain tissue—maybe not using tartrazine, but with another light-absorbing compound. The study investigators did not test if the dye altered cell physiology, Imai says, and the concentration needed is likely toxic.
“I don’t think our current chemical will work for the brain at this concentration, but there are potentially future options,” Ou says. “If we are not able to make it completely transparent, even a slight improvement can be useful.”