Scientists have found a CRISPR-like enzyme with DNA-cutting abilities in eukaryotes, and they say they can program it to edit the human genome.
The new gene editor, called Fanzor, is more compact than the enzymes used in CRISPR technologies, suggesting it could get delivered to cells more easily.
“It can be deployed in cell culture to create genetic models that reflect individual genotypes,” says lead investigator Feng Zhang, a pioneer of CRISPR and investigator at the Massachusetts Institute of Technology (MIT) and Harvard University. “In the long term, Fanzor may be developed into a therapeutic that can be delivered to the brain to modulate the genome.” Zhang and his colleagues described their findings last month in Nature.
The programmable DNA scissors used in CRISPR systems — known as Cas enzymes — were discovered in bacteria and other prokaryotes about a decade ago. They use guide RNAs to pinpoint which sites to edit in genomes.
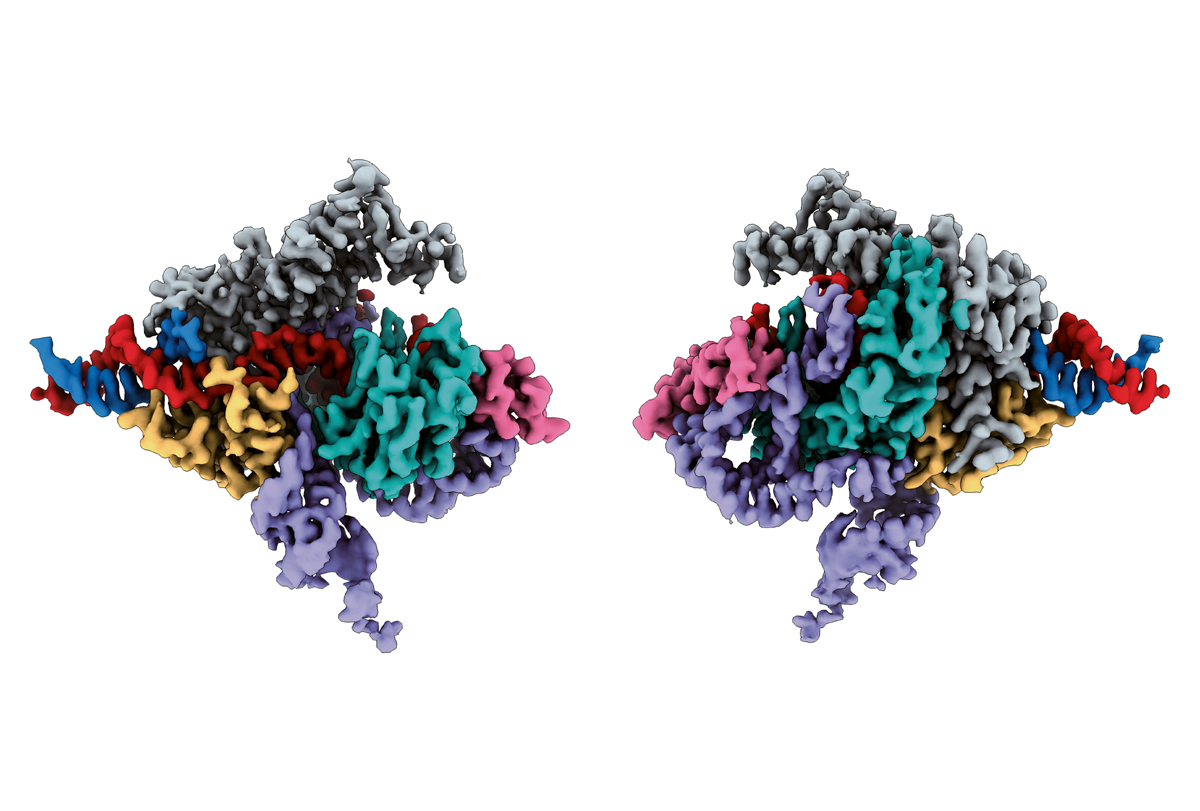
In 2021, Zhang and his colleagues discovered another RNA-guided DNA-cutting enzyme in prokaryotes known as TnpB, from which the Cas12 enzyme likely evolved. In addition, they found that TnpB might also be the ancestor of Fanzor proteins, which were discovered in eukaryotes in 2013 but had unknown functions. This raised the possibility that Fanzors might work as gene editors.
In the new study, the scientists examined Fanzors from four eukaryotes: a clam known as the Northern quahog, as well as a fungus, an algae and an amoeba. They found that Fanzors used guides known as omega-RNAs to target sites in the genome.
“It is quite exciting to see the existence of CRISPR-like proteins in animal cells,” Zhang says. “This work really underscores that there are likely more RNA-guided systems out there in nature that hold future promise for gene editing.”
U
All of the Fanzors except that of the algae were able to edit human embryonic kidney cells grown in culture dishes, with an efficiency of up to 11.8 percent, Zhang’s team found. By contrast, current CRISPR systems can edit human cells with more than 90 percent efficiency, but this is after more than a decade of engineering to improve them. By systematically mutating the fungal Fanzor, the researchers found they could similarly boost its editing efficiency of various sites in the human genome by up to roughly 10-fold.
“One possible practical upside of Fanzors is that, if optimized, they might be more amenable to editing human cells, since they evolved in cells with similar nuclear architectures and structures,” says Ethan Bier, professor of cell and developmental biology at the University of California, San Diego, who did not take part in this research.
Whereas the fungal Fanzor the scientists analyzed is 638 amino acids in length, the most comparable CRISPR enzyme is 1,307 amino acids long, Zhang says. Although Fanzor guide RNAs are longer than those of CRISPR, “overall, the Fanzor system is substantially more compact,” he says. “That has major ramifications for our ability to deliver Fanzor to cells, a key goal for harnessing any gene-editing system for therapeutic use.”
Like CRISPR, Fanzor makes edits by cutting two strands of DNA at once and using a cell’s own error-prone repair machinery to fix these breaks, which might potentially lead to uncontrolled harmful insertions or deletions of DNA. However, the fungal Fanzor did not cleave unintended sites, as some CRISPR systems do, the study shows.
Zhang notes that he and his colleagues were able to modify CRISPR so that it creates more reliably mended single-strand breaks, one at a time, which was later adapted for a technique called prime editing. Future work could engineer Fanzors in a similar way and “make them a valuable new technology for human genome editing,” Zhang says.
I
“It’s remarkable that we are able to find the spread of what we thought were bacterial and archaeal systems to eukaryotes, and for these systems to have remarkably spread through all branches of eukaryotic life,” says co-lead investigator Omar Abudayyeh, a fellow at MIT.
HERMES hosts include amoebae, fungi, plants and animals. “Although these enzymes do occur in animals, they have not yet been found in humans, so we can’t say we have this class of enzymes naturally in our own genomes yet,” say co-lead investigator Jonathan Gootenberg, a fellow at MIT.
Experiments with an amoeba HERMES showed it can be used for gene editing of human embryonic kidney cells. HERMES proteins are typically about 500 amino acids long.
“This size allows easier delivery with certain viral vectors, such as AAV [adeno-associated viruses],” Gootenberg says. “It’s still early days for the efficiency of the nucleases, and they are less established, but there are ongoing efforts to improve their activity.” Abudayyeh notes that future research should also identify the natural roles of these HERMES proteins.