One question long plagued memory researcher André Fenton: How can memories last for years when a protein essential to maintaining them, called memory protein kinase Mzeta (PKMzeta), lasts for just days?
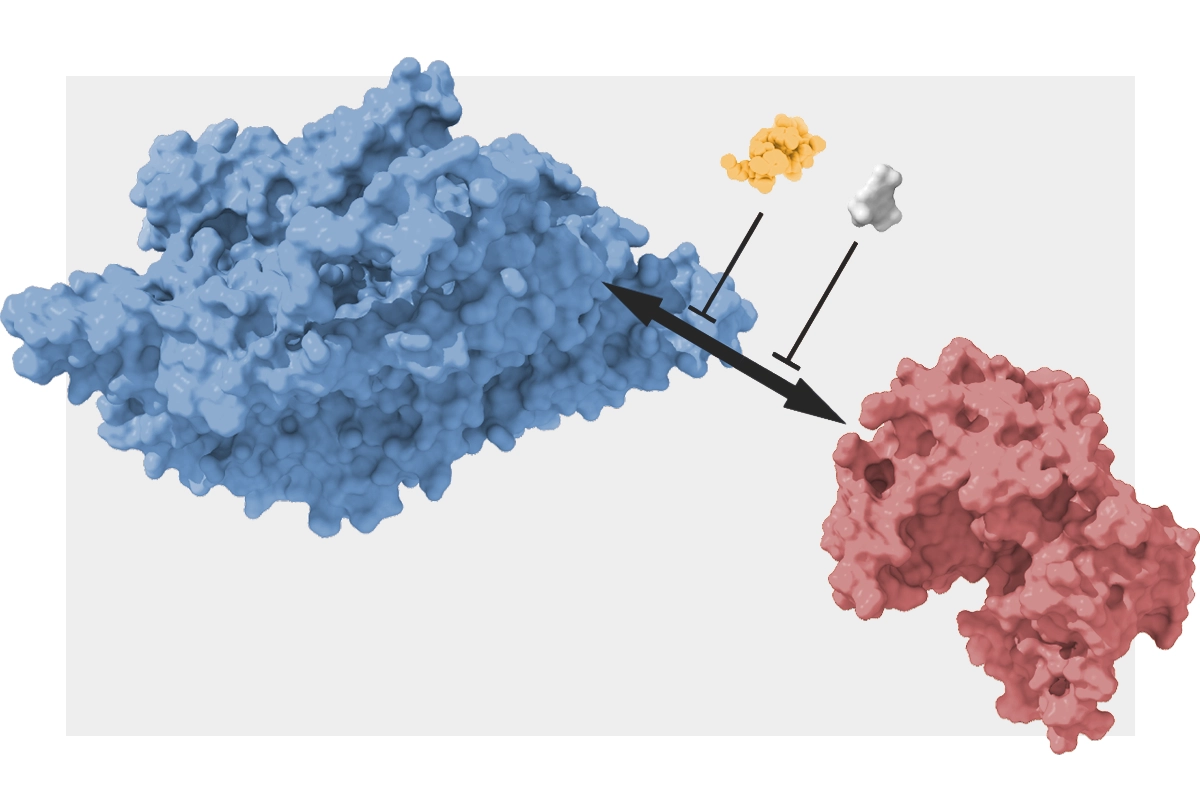
The answer, Fenton now says, may lie in PKMzeta’s interaction with another protein, called postsynaptic kidney and brain expressed adaptor protein (KIBRA). Complexes of the two molecules maintain memories in mice for at least one month, according to a new study co-led by Fenton, professor of neural science at New York University.
The bond between the two proteins “protects each of them,” Fenton says, from normal degradation in the cell.
KIBRA preferentially gloms onto potentiated synapses, the study shows. And it may help PKMzeta stick there, too, where the kinase acts as a “molecular switch” to help memories persist, Fenton says.
“As Theseus’ Ship was sustained for generations by continually replacing worn planks with new timbers, long-term memory can be maintained by continual exchange of potentiating molecules at activated synapses,” Fenton and his colleagues write in their paper, which was published last month in Science Advances.
Before this study, the PKMzeta mystery had two “missing puzzle pieces,” says Justin O’Hare, assistant professor of pharmacology at the University of Colorado Denver, who was not involved in the study.
One was how PKMzeta identifies potentiated synapses, part of the cellular mechanism underlying memory formation. The second was how memories persist despite the short lifetime of each PKMzeta molecule. This study “essentially proposes KIBRA as a solution to both of those—and the experiments themselves are pretty convincing and thorough. They do everything multiple ways.”
P
KMzeta has been widely studied, but its role in memory has been shrouded in controversy for more than a decade, Fenton says. Although early work suggested that PKMzeta is necessary for memory formation, later studies found that they still form in mice missing the gene for PKMzeta.The controversy, Fenton says, forced his team to look for another molecule that might be involved in long-term potentiation. They focused on KIBRA because the scaffolding protein is found in neurons and has been shown to interact with similar kinases in the sea slug.
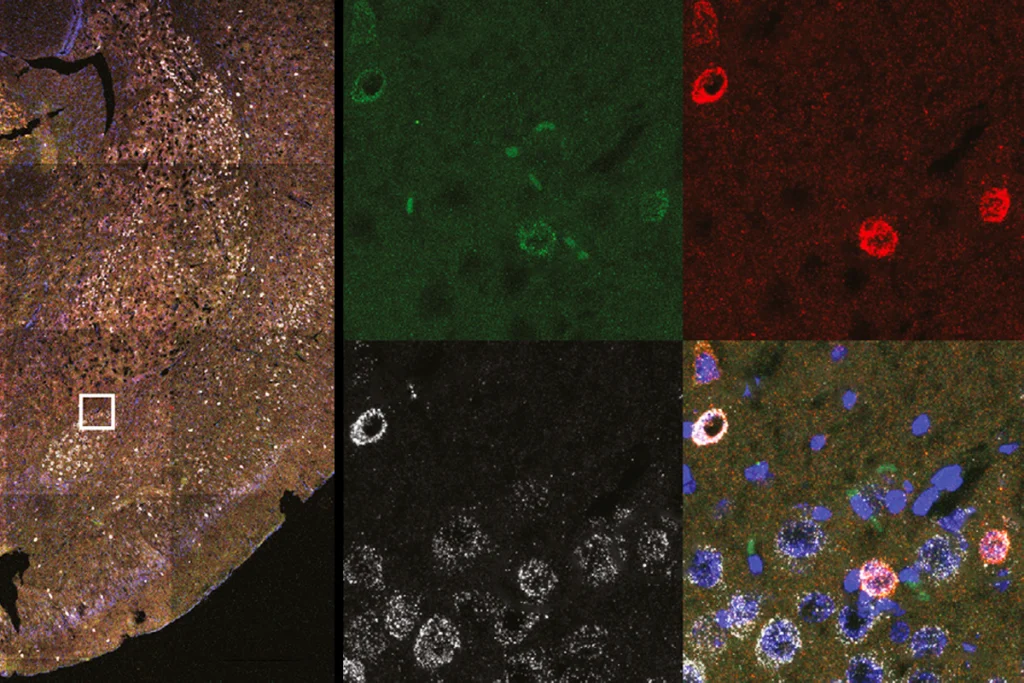
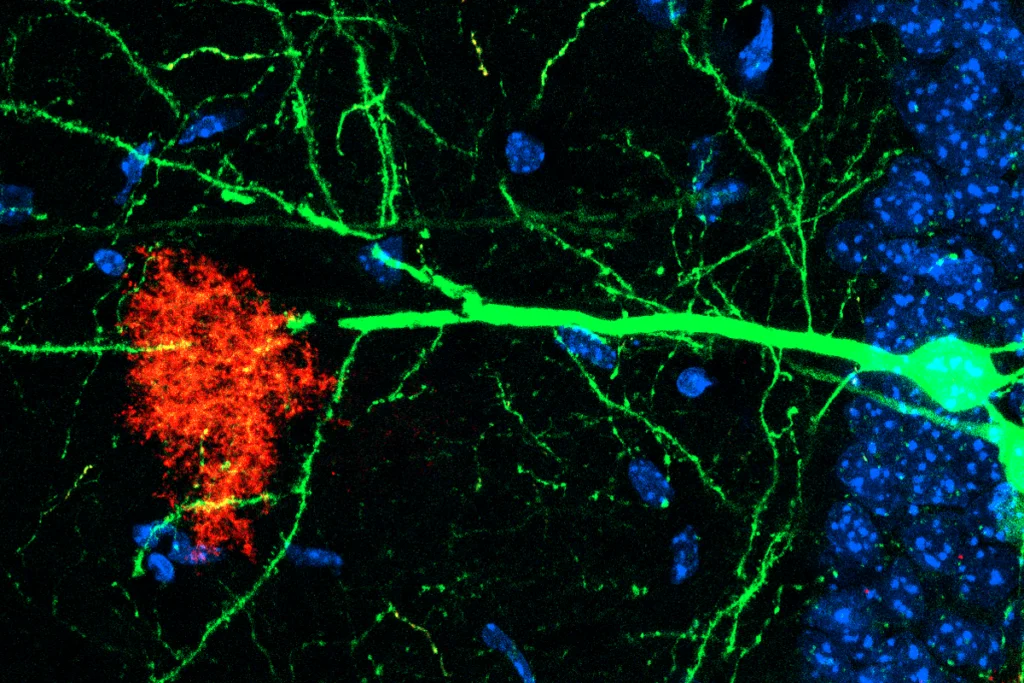
In a common benchtop experiment of memory persistence, electrical stimulation of the CA3 region in mouse hippocampal slices induced complexes of KIBRA and PKMzeta to form in the synapses of the downstream CA1 stratum radiatum region, Fenton’s team found, confirming their suspicions.
“Not only did [KIBRA and PKMzeta] interact, but they interacted in the right places for storing a memory or maintaining [long-term potentiation],” Fenton says.
The excitatory postsynaptic potentials, a proxy for long-term potentiation, across CA1 neurons in the slices remained high three hours after stimulation, the researchers found, but then dipped back down to baseline after treatment with two molecules—zeta-stat and K-ZAP—that block the interaction between KIBRA and PKMzeta.
The two inhibitors also caused wildtype mice to forget their training in two different foot-shock experiments when administered either three days or one month—a time frame longer than the kinase’s typical turnover—after the animals had learned the task.
This result suggests that the KIBRA-PKMzeta complex is crucial for long-term potentiation and for memory maintenance, Fenton says.
E
xperiments repeated in mice engineered to lack PKMzeta showed that neither inhibitory molecule affected the potentiation in the hippocampal slices or the memory of the mice three days after learning a task.That ruled out the possibility that other factors—such as off-target effects of the two inhibitory molecules—caused the depotentiation or memory erasure in the mice. But it raised another question, Fenton says: “So if PKMzeta is so important, and you delete it and you have normal memory and you have normal [long-term potentiation], like, what gives?”
Another kinase, PKCiota/lambda, may step in and bind to KIBRA when PKMzeta is not around, Fenton says. Past work by Fenton and his colleagues has shown that PKCiota/lambda binds to KIBRA at a 10-fold lower rate than PKMzeta does.
This weaker interaction might explain why PKMzeta-null mice did maintain memories, but “the memory is not as good,” Fenton says. For example, in one experiment type, the PKMzeta-null mice re-enter an area where they previously received a mild foot shock more quickly than do their wildtype peers that did not receive the inhibitory molecules, but more slowly than the wildtype mice that received inhibitors, the study showed.
This result answers a question about another inhibitory molecule, the zeta inhibitor protein. ZIP, a 2020 study showed, interrupts long-term potentiation in mice that lack PKMzeta, indicating that memory relies on “a completely different mechanism of action” than PKMzeta, says Rami Yaka, professor of psychopharmacology at the Hebrew University of Jerusalem, who led the 2020 work but was not involved in the current study.
But ZIP is known to broadly target both PKMzeta and PKCiota/lambda, Fenton says. The inhibitors in the current study were specific to PKMzeta and so did not affect PKCiota/lambda.
“There needed to be an explanation for why knocking out PKMzeta allowed memories to persist or [long-term potentiation] to persist,” Fenton says.
How KIBRA becomes “primed to capture PKMzeta” and how KIBRA is attracted to the potentiated synapses remain open questions, says study investigator Panayiotis Tsokas, assistant professor in anesthesiology, physiology and pharmacology at SUNY Downstate Health Sciences University.
The answer might lie in calcium signaling in NMDA channels, which Tsokas says the team is exploring next.