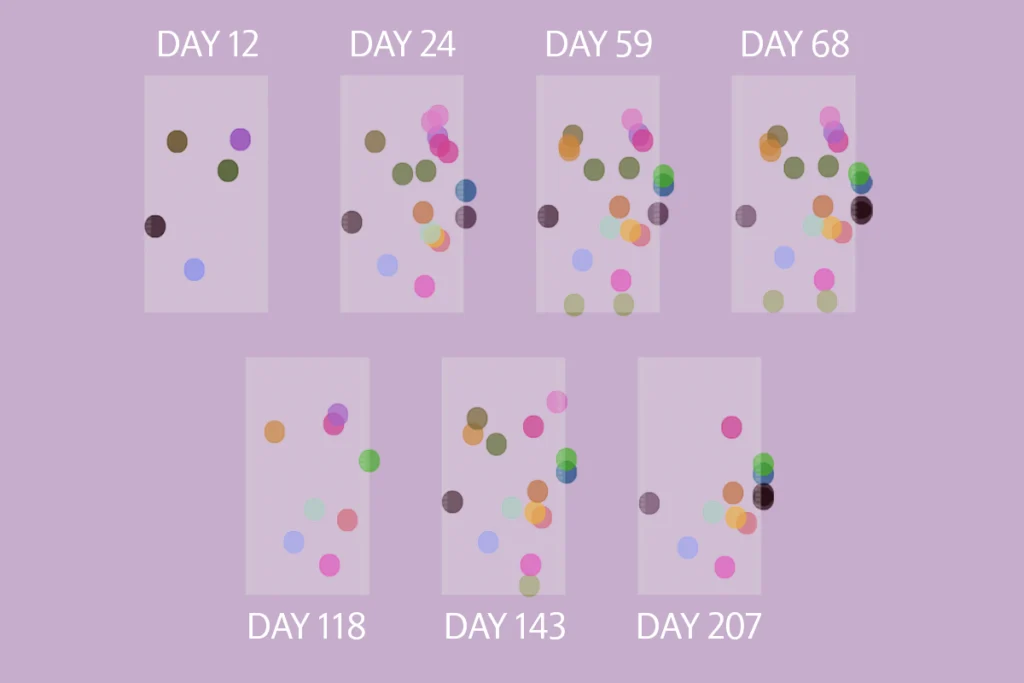
Animal models are invaluable across neuroscience, but they don’t allow us to study human-specific cognitive functions—such as language, abstract reasoning and complex decision-making—outside the human brain. Single-neuron electrophysiology recording offers insight into the neural coding of these abilities at a level of detail that is impossible via noninvasive or less invasive methods. Techniques such as functional MRI and electrocorticography are complementary and equally important in human neuroscience but lack the spatial and temporal resolution to capture the building blocks of neural computation—the single-neuron action potential. By recording from individual neurons in awake, behaving human participants, we gain direct insight into the millisecond-timescale computations that support cognitive functions, informing both fundamental neuroscience and the development of novel clinical applications, from brain-machine interfaces to treatments for neurological conditions.
Because collecting these recordings is so invasive, researchers seeking to study single neurons in humans have long relied on patients already undergoing surgical procedures for epilepsy and other conditions. Neurosurgeons Arthur Ward and Louis Thomas first recorded activity from a single neuron in an awake human 70 years ago, inserting a glass micropipette into a patient’s neocortex during surgery for epilepsy. Glass micropipettes soon gave way to sturdier tungsten electrodes, enabling access to deeper structures, particularly the basal ganglia in Parkinson’s disease, and the ability to track spontaneous activity in individual neurons, including how that activity changed at the onset of a tremor. In 1971, Marcel Verzeano and his collaborators recorded single-neuron activity during a seizure for the first time, using several microwires to increase their chances of recording multiple individual neurons. Like catching a meteor falling on camera, they were fortunate to register the event.
Since then, researchers have developed recording technologies with more electrodes, capable of recording from more cells in more regions for longer periods of time, that can grant greater insight into the inner workings of the human brain than ever before. With stable, longer-term recording capability, researchers can study not just memory and cognitive tasks but also broader brain states, such as wakefulness versus sleep—demonstrating, for example, that hippocampal-cortical interactions during sleep are important for memory consolidation.
N
euroscientists and neurosurgeons now have several technologies at hand for accessing the human brain. The Behnke-Fried electrode, first developed 30 years ago, refined microwire-based electrodes; a commercialized version of the technology remains widely used in epilepsy monitoring. Scientists have leveraged its strengths—access to deep medial temporal structures in an awake and interactive patient, plus hours-long recordings—to explore neural coding. A notable experiment from 2005 identified “concept cells,” also known as “Jennifer Aniston” or “grandmother” cells, in the medial temporal lobe that respond selectively to specific concepts, independent of the sensory modality. A neuron might fire when a patient sees a picture of the actress Jennifer Aniston, hears her name or even imagines her, indicating an abstract representation of the concept rather than a simple visual or auditory representation. More recently, researchers from Ueli Rutishauser’s lab used these same microwire-based electrodes to examine how the human hippocampus encodes abstract representations. This study provides compelling evidence that the human hippocampus encodes cognitive maps and, critically, that rapid, instruction-driven learning can reshape these maps in ways that experience-driven learning in animals cannot.The Utah array, in contrast to deep-penetrating Behnke-Fried electrodes, offers access to the cortical surface. Developed by Richard Normann at the University of Utah and introduced into human research in the 1990s, the pencil-eraser-sized grid houses up to 128 silicon needles, each with a microelectrode at its tip. Since it was invented, the array has been the primary platform for human cortical surface recordings. Because the array moves with the brain surface rather than being anchored to the skull, it can more stably record from individual cells over long time periods, in some cases recording single-neuron spiking activity for years. (It requires connection to a computer through the skin.) Most human research using Utah arrays has been conducted as part of clinical trials developing brain-computer interfaces for people with paralysis, such as in the BrainGate trial, in which arrays implanted in the motor cortex leverage neural firing rates to control computer cursors or robotic arms. Researchers have also used data from these efforts to study neural dynamics of speech and language.
The most recent addition to the human neuroscientist’s toolbox is the Neuropixels probe, a planar array that, thanks to microchip nanofabrication techniques, integrates recording and signal processing on the same piece of silicon. Neuropixels probes pack hundreds of electrodes onto a 1 centimeter shank, expanding the scale of recordings possible and significantly reducing the per-electrode device costs; in intraoperative studies, a single Neuropixels array inserted into the cortex can record more than 100 neurons for tens of minutes. Combined with automated spike-sorting methods, Neuropixels enables detailed spatial characterization of single neurons within specific structures.