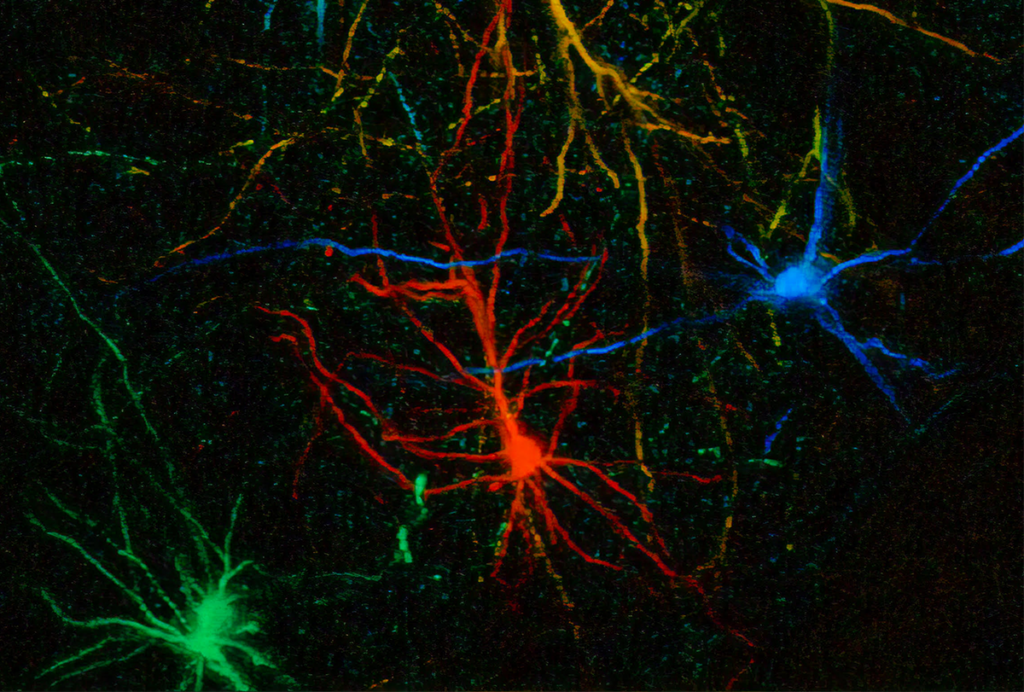
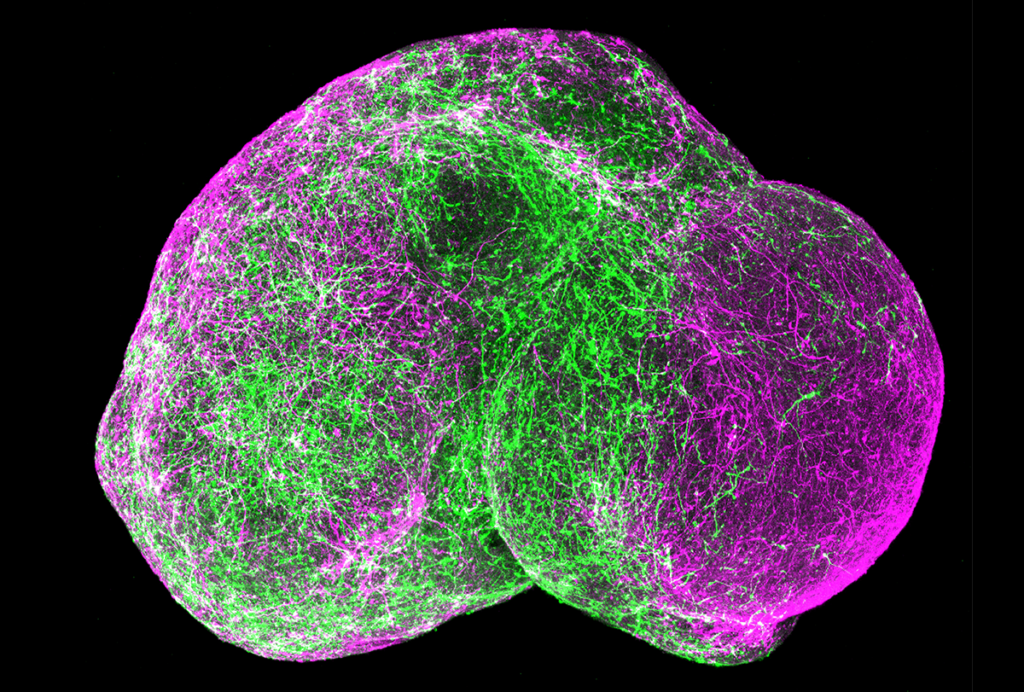
The past two decades—and particularly the past 10 years, with the tool-focused efforts of the BRAIN Initiative—have delivered remarkable advances in our ability to study and manipulate the brain, both in exquisite cellular detail and across increasing swaths of brain territory. These advances resulted from improvements in tools such as optical imaging, chemogenetics and multiprobe electrodes, to name a few. Powerful as these technologies are, though, their invasive nature makes them ill-suited for widespread adoption in human brain research.
Fortunately, our fundamental understanding of the physics and engineering behind noninvasive modalities—based largely on recording, generating and manipulating electromagnetic and acoustic fields in the human brain—has also progressed over the past decade. These advances are on the threshold of providing much more detailed recordings of electromagnetic activity, not only across the human cortex but at depth. And these same principles can improve our ability to precisely and noninvasively stimulate the human brain.
Though these tools have limitations compared with their invasive counterparts, their noninvasive nature make them suitable for wide-scale investigation of the links between human behavior and action, as well as for individually understanding and treating an array of brain disorders.
The most common method to assess brain electrophysiology is the electroencephalogram (EEG), first developed in the 1920s and now routinely used for both basic neuroscience and the clinical diagnosis of conditions ranging from epilepsy to sleep disorders to traumatic brain injury. It’s widely used, given its simplicity and low cost, but it has drawbacks. Understanding exactly where the EEG signals arise from in the brain is often difficult, for example; electric current from the brain must pass through multiple tissue layers (including overlying brain itself) before it can be detected with electrodes on the scalp surface, blurring the spatial resolution. Advanced computational methods combined with imaging data from MRI can partially mitigate these issues, but the analysis is complex, and results are imperfect.
Still, because EEG can be readily combined with behavioral assessments and other functional imaging tools, including MRI and PET, EEG remains a highly valuable means to assess electrical activity and its relationship with behavior and regional brain function. Recent studies linking brain oscillatory activity to widespread hemodynamic and neural fluid flows during sleep demonstrate how integrating EEG with other tools can provide new insights into complex brain behaviors.
The brain stimulation counterpart of EEG is transcranial Direct Current Stimulation (tDCS), in which electric current is applied through the scalp to induce local changes in brain activity. tDCS has many of the same strengths as EEG, including relative simplicity and low cost, enabling its broad use in many laboratory and clinical settings. But similar to EEG, the ability to target specific brain areas for stimulation or inhibition is quite limited. As with EEG, combining additional anatomical information from an MRI or CT scan improves predictions for where the currents will flow, but we are still limited in directing where these target the brain. Nevertheless, tDCS is used clinically to treat depression, neuropathic pain, migraine and other conditions.
M
apping (EEG) or applying (tDCS) electric fields are not the only ways to assess or induce electrical activity in the brain. As James Clerk Maxwell showed more than 150 years ago in the famous “Maxwell’s equations,” electrical and magnetic fields have a direct link; where there is one, there will be the other. So each of the methods above has an important “magnetic” counterpart.Magnetoencephalography (MEG) can detect with great sensitivity focal neural activity in the brain. Unlike electrical signals, MEG signals travel uncorrupted through overlying tissues, making it possible to map neuronal activity with greater spatial precision. This means that MEG is a preferred tool for assessing focal epilepsy, where spatial precision is essential. The same is true for research applications, such as determining the detailed structure of somatosensory maps. Unfortunately, MEG technology is expensive and therefore more difficult to combine with other modalities.
A newer generation of magnetic detectors—so-called optically pumped magnetometers (OPMs)—may help resolve this limitation. They can tolerate a greater degree of participant motion, making it possible to study brain behavior correlates in more natural settings and potentially reducing the costs associated with MEG laboratory setups.
tDCS’s magnetic counterpart is called transcranial magnetic stimulation (TMS). Just as with MEG signals coming from the brain, magnetic fields are not perturbed on their way into the brain, making it possible to target specific brain areas.
Both tDCS and TMS have an important limitation; today at least, both are confined to perturbing the cortex, and neither is adept at stimulating or modulating the brain’s deeper structures. Researchers are exploring promising novel approaches to extend this reach, but currently these methods can modulate deeper structures only by stimulating or inhibiting the cortical sheet and targeting cortical-subcortical network connectivity.
Acoustic stimulation offers an alternative means to modulate brain activity. High-intensity focused ultrasound (HIFU) applies ultrasound energy at high power to heat and destroy abnormal tissues. In the brain, this can include not only tumors, but brain areas disrupted in functional disorders such as Parkinsonian tremors. At lower energy levels, focused ultrasound seems to stimulate neural tissues, though how it does so is not fully understood. Combined with a person’s magnetic resonance or CT anatomical data, ultrasound can deliver targeted energy to specific regions with millimeter precision. And unlike current electromagnetic methods, it can do so at any depth. Though the technology is still in its early stages, its ability to target focally and deep in the brain makes it an attractive approach for both scientific exploration and potential therapeutic applications.
W
hat is missing in this part of the story, for both electromagnetic and ultrasound stimulation, is now less where these fields do their bidding than how. The fundamental biophysics of how electromagnetic and acoustic fields interact with brain tissue to induce neuronal stimulation or inhibition, including in both neurons and surrounding glia, is still far from resolved. We know that these fields can profoundly influence brain activity. But we still need to understand at a much deeper level how this happens if we are to fully harness these tools’ potential for studying and manipulating brain function.The range of applications is as broad as our curiosity about how the human brain works. Clinicians are evaluating how to use more spatially precise TMS to modulate disorders such as treatment-resistant depression by targeting nodes of affected brain networks that lie on the cortical surface. This approach makes it possible to indirectly target deep brain regions by mapping individual connectivity patterns in each person, typically measured using structural or functional MRI.
These same techniques are also being used to map cortical and subcortical connectivity throughout the brain. In other studies, researchers are combining EEG-based measures of oscillatory electrical activity across the cortex with functional imaging to study the relationship between electrical activity, associated hemodynamic changes and neural fluid transport during sleep and other brain states. These efforts are providing insights into the links between the brain’s coordinated electrical activity and the biomechanics of waste disposal.