When functional MRI (fMRI) was first developed 32 years ago, it launched a new field of neuroscience, and tens of thousands of studies have since exploited its ability to localize brain activity. The immediate and unique power of fMRI to show activity changes throughout the entire 3D brain provided a unique (and visually compelling) way to map the spatial properties of brain organization.
Classic fMRI studies have generated many insights. But behind the scenes, rapid advances in MRI physics have enabled the development of powerful new methods that are capable of much more than many neuroscientists realize and that offer novel information about brain physiology.
A key recent advance in fMRI is speed: Data can now be acquired about 10 times faster than was possible decades ago. A classic fMRI study might have generated a brain image every 3 seconds or so, but it’s now possible to image the whole brain in less than 300 milliseconds. This acceleration transforms fMRI into a tool that can measure activity not just in space, but in time. Fast imaging can measure temporal sequences of neural activity spaced just a few hundred milliseconds apart, tracking propagating dynamics throughout the cortex and subcortex. This powerful tool for noninvasive human neuroimaging introduces a unique capability: No other method can achieve subsecond whole-brain imaging in mammals.
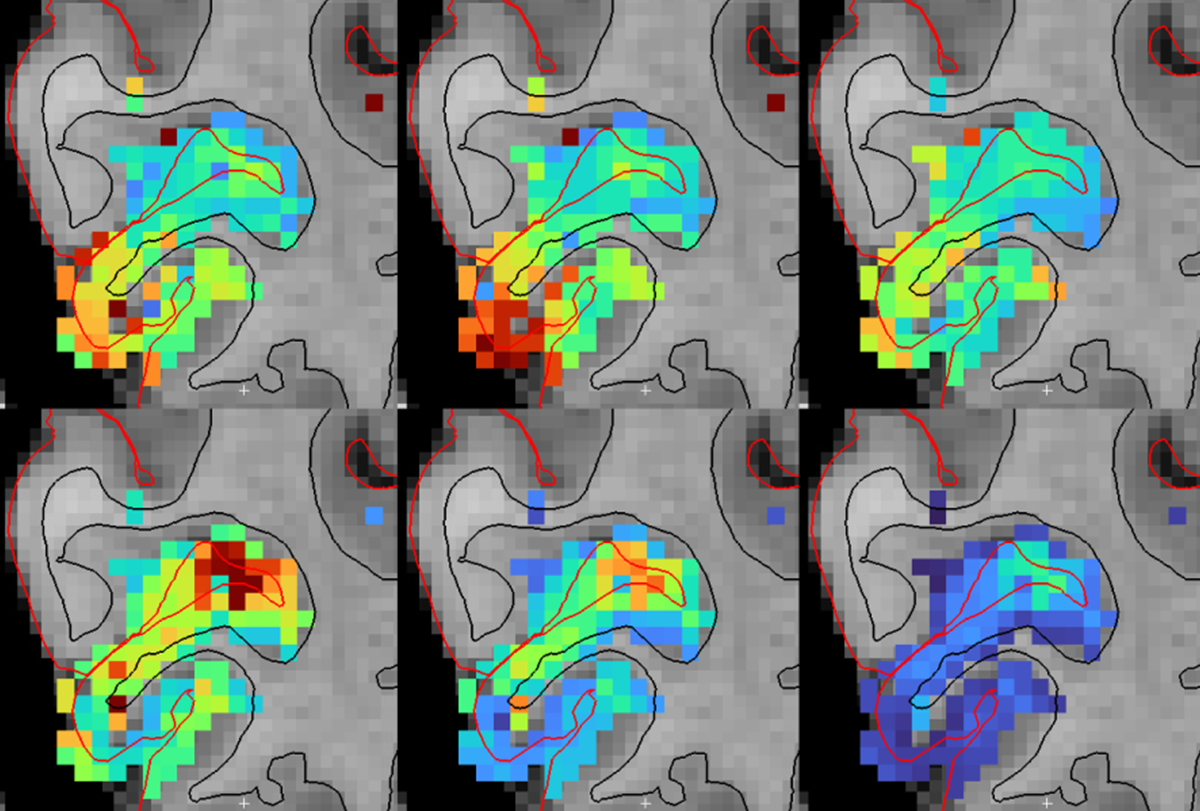
The spatial resolution of fMRI is also improving, with recent human functional imaging studies able to resolve voxel sizes of less than 500 microns. This spatial resolution can distinguish specific structures, such as deep and superficial cortical layers, enabling fMRI to explore laminar circuits. A particular advantage of fMRI over other methods is its ability to image throughout the entire brain. Even with fMRI, subcortical imaging has been relatively limited in the past because of weaker signals in this region and the tiny size of nuclei in the subcortex. But enhanced spatial resolution can now resolve many tiny subcortical nuclei, for example throughout thalamus and brainstem, enabling the measurement of activity in dozens of subcortical brain regions simultaneously. Because the subcortex is the key controller of fundamental functions such as sleep, arousal, autonomic function and mood, this increased resolution holds exciting promise for the neuroscience of brain and body state regulation.
A third exciting development is the new contrast methods emerging in MRI, which measure aspects of anatomy and physiology that were previously out of reach. Though neuroscientists typically use fMRI to measure changes in blood oxygenation (to infer neural activity), MRI contains diverse other signals as well. fMRI scans can be modified to measure cerebrospinal fluid flow, for example, to explore how neural circuits regulate brain fluid dynamics. Pulse sequences that measure blood volume (instead of oxygenation) can disentangle signals from deep and superficial cortical layers, providing laminar precision in human imaging. Magnetic resonance (MR) spectroscopy can be used dynamically over time to noninvasively measure metabolites in brain tissue. And MRI can be combined with other modalities, such as EEG or PET imaging, for example to simultaneously map brain-wide hemodynamics and dopamine receptor occupancy, adding neuronal and chemical specificity.
T
hese new methods have been used in several cutting-edge studies to look at the brain in qualitatively new ways. But they haven’t yet replaced the bread-and-butter conventional methods in many neuroimaging studies. Why? Several factors play a role.First, MRI is an expensive technology, even more so when using the latest hardware. Some innovations involve new hardware that may be available only at specific sites, so we need funding mechanisms to support partnerships between technology developers and neuroscientists, so that the latter can use these new tools to address biological questions. A few sites now use this model, and expanding it could accelerate neuroscience broadly. But cost isn’t the only limiting factor. Many new MRI technologies are “pulse sequences,” or novel programs to acquire images. These pulse sequences can work on scanners that are already available and can often be transferred to existing MRI research centers without the need for new equipment—but they require technical expertise to implement successfully.
This issue points to the second and larger barrier: the way our scientific communities are structured and the gaps in knowledge across different fields. Neuroscientists and MR physicists often operate in separate communities and attend separate conferences, slowing translation and limiting the impact of new methods. Neuroscientists sometimes underestimate MRI, and in some cases, word simply hasn’t yet spread about all the new things it’s now possible to do. They may also hesitate to embrace new MRI technologies, which are often unstable, difficult to use and yield complex data that are not fully understood and need biological validation. At the same time, MR physicists may not know the biological questions their methods could address. Close collaboration between these developers and users is essential to get new technology to yield meaningful biology.
Finally, using fMRI-based methods to make accurate statements about neural activity requires precise biological models of the signals we measure. Because most fMRI methods measure hemodynamic signals, understanding what neurons are doing depends on understanding neurovascular coupling: We need to define the relationship between neural activity and hemodynamic signals captured in MRI. Recent research makes clear that although neurovascular coupling is complex and variable, it can also be surprisingly precise and contains multiple dimensions of information. However, software for analyzing fMRI data often defaults to built-in, simplified assumptions about the hemodynamic response that are valid in some contexts but not others. Improving these models and incorporating the biology of neurovascular dynamics into fMRI analyses needs to be a priority for fully enabling the potential of these new scanning paradigms.
Looking to the future, MRI scanners continue to evolve, yielding a new era of machines with more powerful gradients and previously unattainable magnetic field strengths for human imaging. For example, the University of California, Berkeley, has constructed the NexGen 7T, a 7 Tesla scanner that enables ultra-high resolution imaging, and Massachusetts General Hospital turned on a 3 Tesla scanner with advanced gradients—the Connectome 2.0—last year, which is now imaging intricate details of anatomical circuitry in people. A 10.5 Tesla scanner at the University of Minnesota recently yielded human brain functional images; an 11.7 Tesla scanner is now online at NeuroSpin in Paris; and the first ever 14 Tesla scanner for humans is slated for construction in the Netherlands in the coming years.
These new scanners promise to bring previously invisible anatomy and physiology into view, and they herald another accelerating era of human neuroimaging ahead, for those who want to seize it. Collaboration between engineers and neuroscientists, as well as a community effort to develop and validate new techniques, will be essential to translate this hardware into new biological insight. In the meantime, the current generation of MRI technology already offers a wealth of opportunities that can be exploited by neuroscientists to measure new kinds of information about the human brain.