New method reignites controversy over brain clearance during sleep
Tracers injected directly into mouse brain tissue instead of the cerebrospinal fluid show that brain clearance slows during sleep and under anesthesia, according to a study published last week—but proponents of the glymphatic system theory take issue with the technique.
A new study suggests that the brain clears less waste during sleep and under anesthesia than while in other states—directly contradicting prior results that suggest sleep initiates that process. The findings are stirring fresh debate on social media and elsewhere over the glymphatic system hypothesis, which contends that convective flow of cerebrospinal fluid clears the sleeping brain of toxins.
The new work, published 13 May in Nature Neuroscience, proposes that fluid diffusion is responsible for moving waste throughout the brain. It uses a different method than the earlier studies—injecting tracers into mouse brain tissue instead of cerebrospinal fluid—which is likely a more reliable way to understand how the fluid moves through densely packed neurons, says Jason Rihel, professor of behavioral genetics at University College London, who was not involved in any of the studies on brain clearance.
The findings have prompted some sleep researchers, including Rihel, to question the existence of a glymphatic system and whether brain clearance is tied to sleep-wake states, he says.
But leading proponents of the sleep-induced clearance theory are pushing back against the study’s techniques. The new study is “misleading” and “extremely poorly done,” says Maiken Nedergaard, professor of neurology at the University of Rochester Medical Center, whose 2013 study on brain clearance led to the hypothesis of a glymphatic system. She says she plans to challenge the work in a proposed Matters Arising commentary for Nature Neuroscience.
Inserting needles into the brain damages the tissue, and injecting fluid, as the team behind the new work did, increases intracranial pressure, says Jonathan Kipnis, professor of pathology and immunology at Washington University School of Medicine in St. Louis. Kipnis and his colleagues published a study in February in support of the glymphatic system hypothesis that suggests neural activity facilitates brain clearance.
“You disturb the system when you inject into the brain,” Kipnis says, “and that’s why we were always injecting in the CSF.”
A
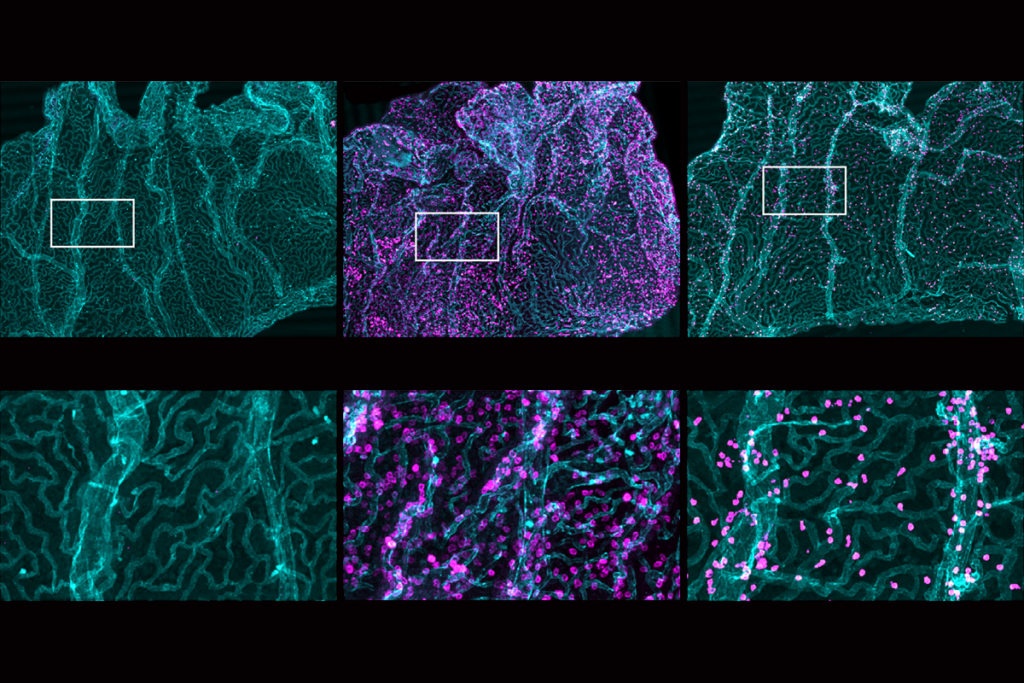
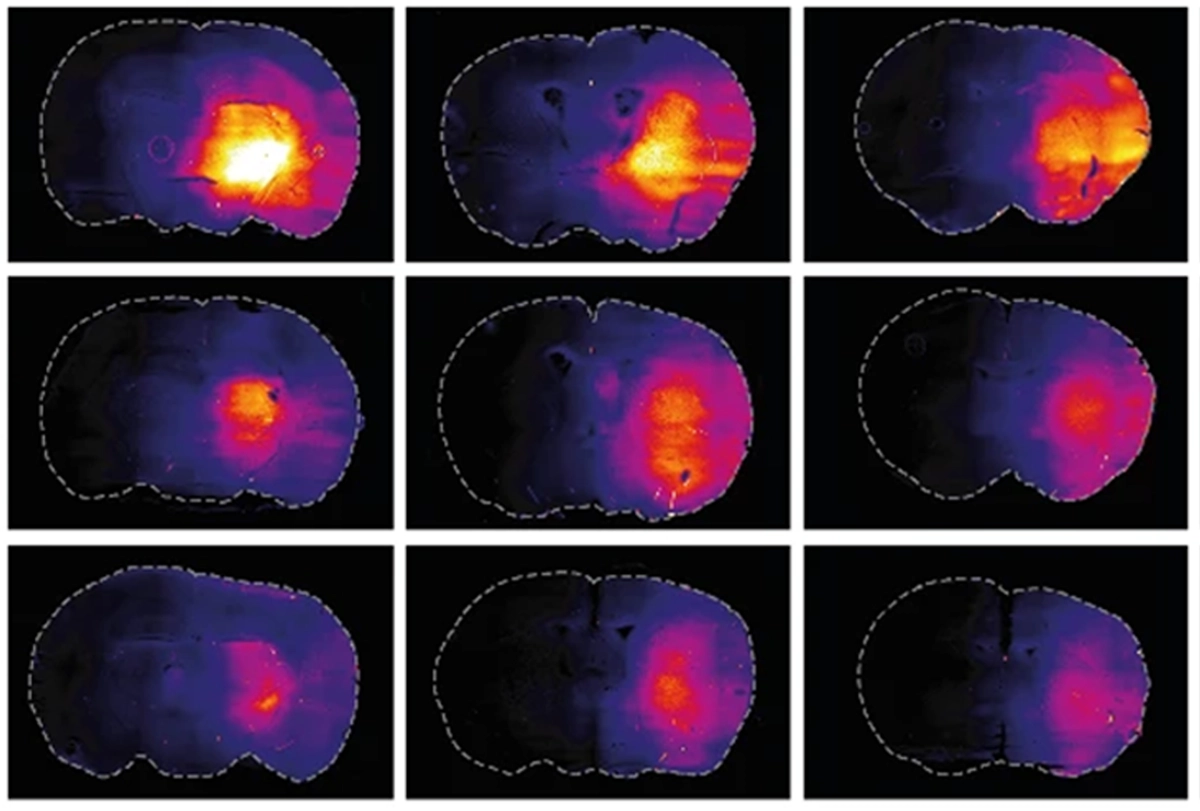
t first, the glymphatic theory “looked like an extremely good idea” to explain why sleep is necessary, says Nick Franks, professor of biophysics and anesthetics at Imperial College London and lead investigator of the new work. Past glymphatic studies measured waste clearance during sleep and other states by introducing markers into an animal’s cerebral spinal fluid and tracking how quickly the markers moved into the brain. An increase in fluid flow into the brain, the 2013 study reported, reflects an increase in the volume of the interstitial space, allowing for more efficient waste clearance through convective fluid flow.But Franks wanted to use a more direct measure of fluid movement in the brain that “did not depend on that bit of logic,” he says. He and his colleagues injected a fluorescent dye through an implanted cannula into the caudate putamen brain region in mice. An optical fiber implanted roughly 3 millimeters away in the frontal cortex tracked the concentration of fluorescent markers as the mice entered different states of wakefulness or sedation during 12-hour periods of light or dark.
Bright light delivered by the optical fiber into the caudate putamen photobleached the dye there, enabling the researchers to measure how quickly unbleached dye reentered the area over 24 hours and to calculate a “diffusion coefficient” in the brain. And this diffusion coefficient matched that measured in other experiments in agarose gel and mouse brain slices.
The diffusion coefficient stayed the same whether the animals were awake or in non-REM sleep, REM sleep, light or dark states or dexmedetomidine sedation, suggesting that the dye moves through the cortex by diffusion, not because of changes in convection as the glymphatic hypothesis predicts, Franks says.
Franks and his team also measured the concentration of a small dye—several hundred daltons smaller than that used in prior work on glymphatic clearance—for 12 hours after the mice were given anesthesia or saline, or during 5 hours of wakefulness or the subsequent 12 hours of sleep. The concentration of dye to arrive at the optical fiber was lower in animals after saline injections or during awake periods than under anesthesia or during sleep. And mouse brain slices showed more dye when harvested during sleep or under anesthesia.
Taken together, the results show that dye diffuses through the brain independent of an animal’s state, Franks says, and the brain clears more dye during wakefulness or after saline than during sleep or while under anesthesia.
“Relatively speaking, more [dye] was retained during anesthesia and sleep—and that was the exact opposite of what the glymphatic hypothesis would predict,” Franks says.
T
he new paper used many of the techniques incorrectly, says Nedergaard, who says she plans to elaborate on her critiques in her submission to Nature Neuroscience. Injecting straight into the brain, for example, requires more control animals than Franks and his colleagues used, to check for glial scarring and to verify that the amount of dye being injected actually reaches the tissue, she says. The cannula should have been clamped for 30 minutes after fluid injection to ensure there was no backflow, she adds, and the animals in the sleep groups are a model of sleep recovery following five hours of sleep deprivation, not natural sleep—a difference she calls “misleading.”“They are unaware of so many basic flaws in the experimental setup that they have,” she says.
More broadly, measurements taken within the brain cannot demonstrate brain clearance, Nedergaard says. “The idea is, if you have a garbage can and you move it from your kitchen to your garage, you don’t get clean.”
There are no glymphatic pathways, Nedergaard says, that carry fluid from the injection site deep in the brain to the frontal cortex where the optical measurements occurred. White-matter tracts likely separate the two regions, she adds. “Why would waste go that way?”
Technical issues, such as intracranial pressure or method of injection, are valid concerns that make it difficult to evaluate the new experiments, Rihel says. But the work’s mathematical modeling of diffusion and careful testing of diffusion techniques in gel are compelling. The results also align with prior theoretical models that suggest diffusion, not convective flow, explains brain clearance, he says. “And if you don’t have this convective flow, then the glymphatic system, at least as it’s conceived, isn’t needed.”
Different molecules might clear from the brain at different rates or in different states, Rihel says. The new study used inert dyes, whereas some prior glymphatic research used biologically relevant molecules, such as amyloid beta, he says.
Even though the new study appears to contradict the glymphatic work, it could just be a different interpretation of the same results, Franks says. The fact that markers injected into cerebrospinal fluid penetrated farther into the cortex during sleep and under anesthesia in the earlier work “can be accounted for equally well by reduced clearance,” he says.
The glymphatic model is more than a decade old, Nedergaard says, adding, “Of course it has to be revised; you always simplify a model, and we are totally up for revising models.” But to claim that the glymphatic hypothesis is wrong, she says, requires replicating the original method to show why it’s wrong.
The idea of a flipped interpretation “just turned things upside down,” says Ali Amidi, associate professor of psychology and behavioral sciences at Aarhus University, who was not involved in the study. “And it’s not often that you see that kind of difference in interpretation of the same outcome.”
To settle the difference in interpretation between sleep-induced studies and the new work calls for a better tool, Kipnis says—specifically, a genetically modified mouse model in which a tracer could be induced at a particular time in a specific brain region. “And now if anybody thought that we solved the problem of sleep, or we solved the problem of brain clearance, I mean, that’s very naive,” he says.
Franks says he expects pushback on this work, which is one reason he has not presented it at conferences—to avoid getting involved with “the zeitgeist.”
“You don’t want people telling you you’re wrong,” he says. “Step back from it. Publish your paper, do the best you can, and then see what happens.”
Recommended reading

Maiken Nedergaard’s power of disruption

During decision-making, brain shows multiple distinct subtypes of activity
Explore more from The Transmitter
Microglia’s pruning function called into question

Rousing a ‘new era’ of hibernation research