Octopus arms may literally have a mind of their own. Each limb contains its own version of a spinal cord, called an axial nerve cord, and these cords collectively harbor most of the animal’s neurons. In some instances, the arms can process sensory information and initiate motor actions on their own, without input from the brain.
But a dearth of molecular tools has blocked deeper insights into the neural circuity of octopus arms. “You need to know not just what types of cells are there but where to find them,” says Robyn Crook, associate professor of biology at San Francisco State University.
To close this gap, Crook and her team built a 3D map of the location and molecular identities of neurons in the axial nerve cord, plus a map of the structure of blood vessels, muscles and other tissues in the arm, published today as a pair of papers in Current Biology. Crook says she hopes the characterizations will “start to facilitate more functional studies.”
The datasets are “going to be very, very valuable down the road,” says Rhanor Gillette, professor emeritus of molecular and integrative physiology at the University of Illinois Urbana-Champaign, who was not involved in the work. Crook’s team, he adds, has created “a very nice reference for future electrophysiological and histological investigations.”
F
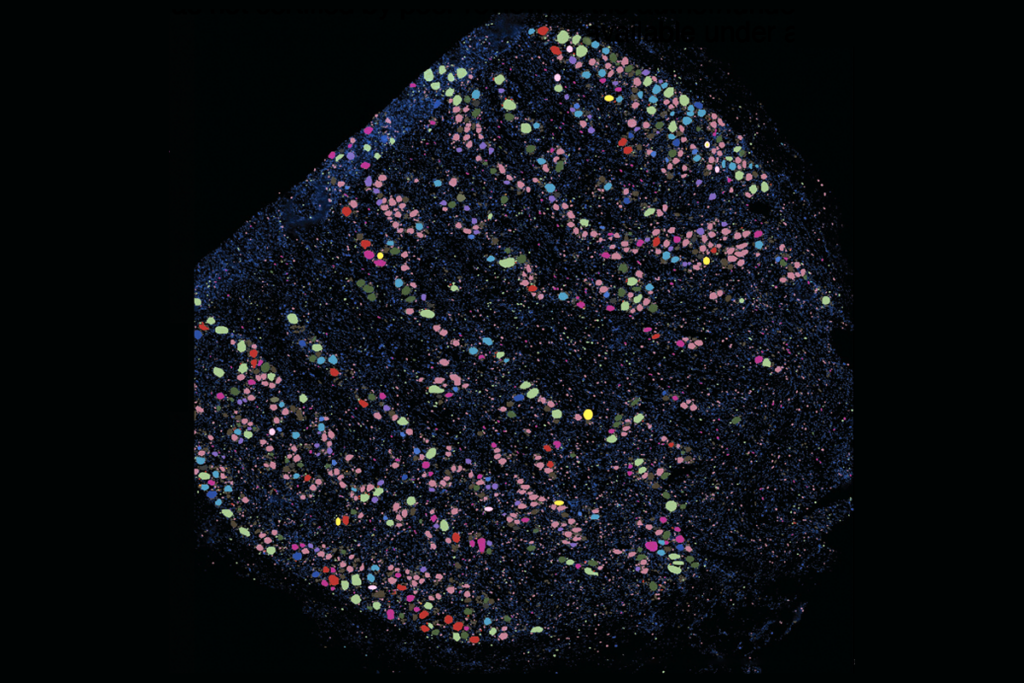
or one of the maps, Crook and her team used block-face electron microscopy to take serial scans of tissue from the base and tip of an arm from Octopus bocki, or the pygmy octopus, and created a 3D rendering of the two sections.The arm is built of “repeating anatomical motifs” oriented around each sucker, the team reported. The axial nerve cord consists of a strand of cell clusters that zigzag from left to right, in tandem with the distribution of suckers. The cord looks more like a strand of Christmas lights than a straight line, says study investigator Diana Neacsu, a former graduate student in Crook’s lab and incoming doctoral student at KU Leuven.
Neacsu also observed tissue tracts connecting four intramuscular nerve cords around the perimeter of the arm. It is not clear what type of cells make up the tracts, because the map provides only morphological data, Neacsu says. The tracts, called oblique connectives, were first described in a 1908 thesis by a doctoral student at the University of Paris. “But this is the first time that we have a chance to actually see them in their full trajectory as they wrap around the arm, which is pretty amazing,” Crook says.
Crook says she has already shared the dataset with other cephalopod researchers, including Trevor Wardill, associate professor of ecology, evolution and behavior at the University of Minnesota. Wardill is working on a comparison of muscles in different cephalopod species, he says, and the electron microscopy data were “quite a good leg up” because of the high resolution.
I
n the second map, Crook’s group sequenced the neurotransmitter mRNA expressed in each axial nerve cord cell and built a 3D reconstruction of slices from the base and tip of the arm. The sequencing method they used, called hybridization chain reaction, captures only a select subset of transcripts but preserves the location information, says study investigator Gabrielle Winters-Bostwick, a postdoctoral fellow in Crook’s lab. “This is really the first time we’ve been able to see how the different cell types are arranged in 3D in the arm.”