A growing tool kit of viruses makes it possible to express genes in select cell types of the brain and spinal cord. Data on some of the adeno-associated viruses (AAVs) are provided in the Allen Institute’s Genetic Tools Atlas, which launched in September, and some of the viruses are available to buy. The tools could help reveal how cell types contribute to cortical functions and neurological conditions, according to a series of preprints posted on bioRxiv in the past 14 months.
Through their systematic and careful work, researchers at the Allen Institute have created viruses to target almost any cell type in any brain region, says Botond Roska, founding director and senior group leader at the Institute of Molecular and Clinical Ophthalmology Basel, who was not involved in the work. “It’s very, very significant,” he adds.
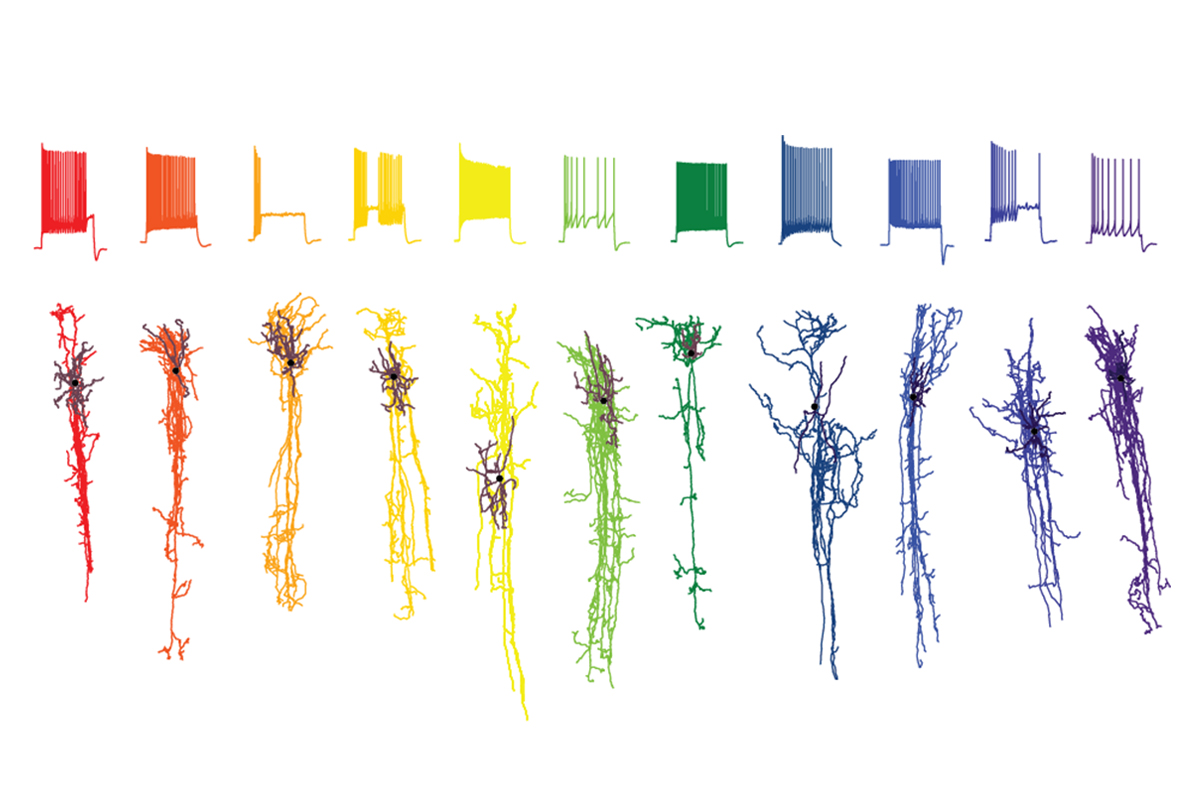
The Allen Institute engineered its first batch of these AAVs in 2021—selective for excitatory projection neurons and neurons that express parvalbumin—and has been expanding the collection ever since. The most recent additions include viruses that target various glial cells and additional neuronal subtypes in the cerebral cortex, striatum and spinal cord.
Each virus is equipped with a short genetic regulatory element called an enhancer, which activates genes by recruiting proteins found in select cell types. To identify these cell-type-specific enhancers across the mouse central nervous system, the institute’s researchers combined data from ATAC-seq—a method for detecting accessible regions of the genome—with single-nuclei sequencing data that identifies cell subtypes based on similarities in gene expression.
The group identified multiple candidate enhancers and packaged each one into an AAV so that it controlled the expression of a gene for a fluorescent protein, and then they screened the AAVs in mice. The most promising viruses—those that express almost all of the fluorescent protein in only one cell type—were further tested and often found to also be effective in rats and non-human primates.
“I
t’s a remarkable repository,” says Ann Graybiel, professor of brain and cognitive sciences at the Massachusetts Institute of Technology’s McGovern Institute, who was not involved in the work. They “are already having highly exciting implications for evolutionary biology,” she says.For example, by optimizing expression of an enhancer-AAV specific to GABAergic neurons, researchers have cataloged diverse GABAergic cell types in brain tissue removed from people during surgery. Comparing their list with the types of GABAergic neurons found in mice, the team uncovered cells in the human brain that are absent in rodents. Such studies could provide “a better understanding of what makes us uniquely human,” says Jonathan Ting, associate investigator at the Allen Institute for Brain Science, who helped develop the tool kit.
And the same GABAergic enhancers have been used in two viruses that each carry a portion of SCN1A, a gene too large to fit into a single viral vector. Mutations in the gene, which codes for a sodium channel expressed in inhibitory interneurons, contribute to a severe form of epilepsy called Dravet syndrome. Mice modeling the condition usually die prematurely, but when they were treated with both SCN1A viruses, all animals survived to adulthood and showed fewer seizures, according to an unpublished study.
F
or scientists investigating how select cell types contribute to behavior, enhancer-AAVs can sidestep the time-consuming process of breeding transgenic mice. “You really simplify the paradigm for doing these types of studies,” Ting says.And viruses provide an element of temporal control that’s lacking in transgenic animals, says Tanya Daigle, assistant investigator at the Allen Institute for Brain Science, who helped to build the AAVs. Although a transgene is expressed from conception, scientists can use viral tools to express genes in specific cell types at a precise stage of an animal’s life. This could “provide the fine-tuning needed for certain experiments, Daigle says.
What’s more, transgenic mice aren’t always the most appropriate model organism. Rodents don’t naturally develop the plaques and tangles associated with Alzheimer’s disease, for example, but scientists use them anyway because the genetic tools to investigate the condition in them are established, says Andreas Pfenning, associate professor of computational biology at Carnegie Mellon University, who was not involved in the work. “What would be really cool is to move beyond mice and into these more appropriate models for certain things,” Pfenning says.
The development of gene therapies also requires research to move beyond mice and into primates. In fact, most of the enhancer-AAVs developed for mice show the same cell-type specificity in macaques and human neurosurgical tissue. One such tool stitches together two enhancers to simultaneously target both upper and lower spinal-cord motor neurons implicated in amyotrophic lateral sclerosis (ALS) in the macaque brain. Combining this tool with capsids designed to penetrate the blood-brain barrier while avoiding the liver could one day yield a gene therapy for ALS that has minimal side effects, Daigle and Ting say.
Yet, for some experiments at least, transgenic mice have the upper hand. Because cells absorb varying amounts of viral particles, or none at all, it’s often difficult to interpret experimental results, Pfenning explains. For instance, if an enhancer-AAV designed to inhibit a specific type of brain cell reduces behavior by 50 percent, that could mean that some of the targeted cells are still active, or that other circuits are involved. “It’s hard to make these absolutist claims if you’re not completely ablating or inhibiting a population,” Pfenning says.
Rather than replace transgenic mice, the viruses could be used in combination, or to plug the gap where transgenic animals for a specific cell type don’t exist, the researchers say. This is where Ting says he and his colleagues plan to focus their efforts next, by supplementing transgenic mice with enhancer-AAVs to get increasingly targeted manipulation of specific brain circuits.
“I don’t think its productive to think about using the enhancer viruses on their own. It’s about how they can be leveraged to extend what we already know and dive deeper,” he says.