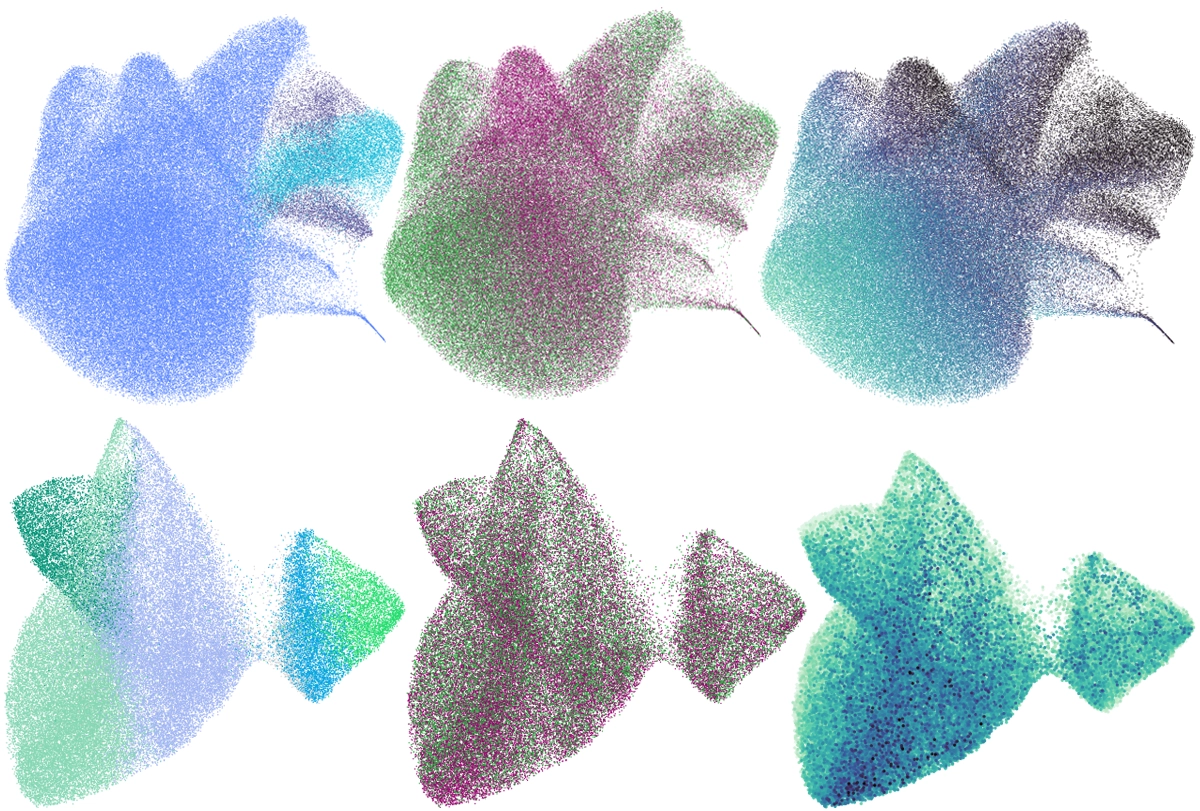Steven McCarroll just wanted to compare how different cell types express genes in people with and without schizophrenia.
But when he sequenced the transcriptomes of more than 1 million cortical cells from 191 postmortem brains, what leapt out from the data went far beyond his simple case-control comparison: Astrocytes and neurons from all of the brains coordinate their expression of certain genes needed for healthy synapses, a relationship the team dubbed the Synaptic Neuron-and-Astrocyte Program (SNAP) and described in a paper published in Nature today.
“The data led us to something much more exciting and surprising than what we set out to do,” says McCarroll, professor of biomedical science and genetics at Harvard Medical School.
SNAP is an intricate dance, McCarroll and his colleagues found: The more a person’s neurons express synaptic genes, so too do their astrocytes, but this coordination wanes in older people and those with schizophrenia.
Because astrocytes — a type of glial cell — and neurons are in constant communication and the findings are correlational, it’s unclear which cell type choreographs this dance. But other evidence suggests that astrocytes take the lead, says Stephen Quake, professor of bioengineering at Stanford University, who was not involved in McCarroll’s work.
In mice trained to fear a foot shock, for example, neurons involved in memory formation express neurotensin, whereas astrocytes express a receptor for it, Quake and his colleagues reported last month in Nature. But when they inhibited the animals’ astrocytes during fear training, the mice performed worse on memory tests, suggesting those cells play an active role in long-term memory formation, Quake says — and govern the relationship McCarroll found.
“It doesn’t surprise me at all,” Quake adds. “In diseases of memory, you’re seeing changes in the astrocytes, because the astrocytes are required for memory. That’s what we showed.”
N
eurons have long been considered the main players in the brain. But with the advent of single-cell sequencing 15 years ago, researchers gained the ability to study other cell types in far more detail.Around the same time, Alzheimer’s disease researchers began to focus on astrocytes, says Nicola Allen, associate professor of molecular neurobiology at the Salk Institute for Biological Studies. The gene most strongly linked to Alzheimer’s disease, APOE, is expressed largely by astrocytes.
Together, these trends led to discoveries that connected astrocytes with healthy cognition, aging and neurodegeneration, Allen says. And that role shows up in the genes the cells express: For example, in aging mice, astrocytes boost expression of synapse-pruning genes, which are necessary in early-life development but may be destructive to an adult brain, Allen showed in 2018.
In organoid models of Parkinson’s disease, in which a gene necessary for clearing disease-causing inflammatory molecules is turned off, astrocytes expressing that gene restore normal functioning to neurons, another team reported in January.
And astrocytes, along with neurons and microglia, express genes associated with age-related structural changes observed in brain scans of nearly 800 people, according to another study published in January.
“It’s just becoming very clear that it’s not just neurons that change in aging,” Allen says. “It’s also the glial cells.”
M
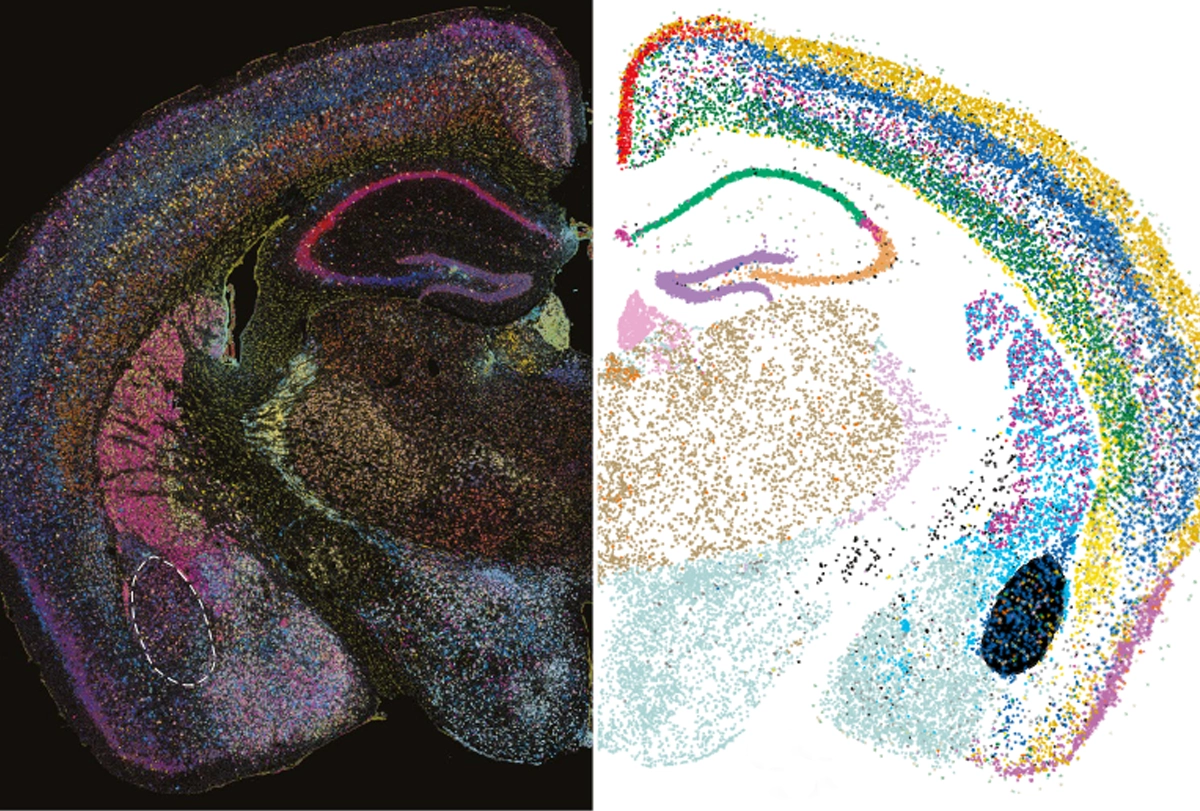
cCarroll and his team analyzed gene expression in 1.2 million cortical cells from 191 people aged 22 to 97 years, 94 of whom had schizophrenia. The brain tissue came from the Harvard Brain Tissue Resource Center/NIH NeuroBioBank.Using a type of machine learning called latent factor analysis, the researchers identified patterns of gene expression across cell types that always increase or decrease together, suggesting they are driven by a common mechanism, or “factor.” They found 10 such factors, and changes in one, called LF4, correlate with schizophrenia. LF4 is less present in cells from older people than in those from younger ones, regardless of whether or not they had schizophrenia.
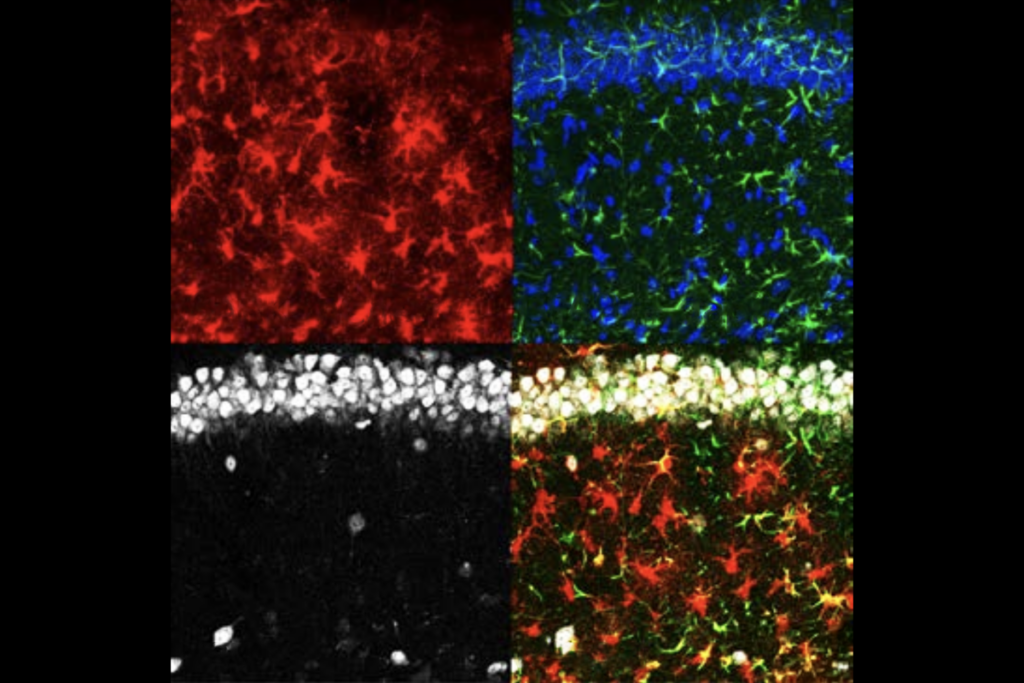
LF4 is most active in neurons and astrocytes. In neurons, LF4 encompasses genes necessary for synapse function. In astrocytes, though, the factor involves genes that make cholesterol and fatty acids, which are necessary for healthy synapses, as well as genes needed to soak up excess neurotransmitters from the space around neurons, one of astrocytes’ key roles.
The more astrocytes expressed these genes, the more neurons expressed synaptic genes, the researchers found — the basic principle of SNAP.
“This advance is looking at this network of perturbations and how it might signal between the different cells,” says Joel Blanchard, associate professor of neuroscience at the Icahn School of Medicine at Mount Sinai and lead investigator on the Parkinson’s organoid study. “I think that’s a really nice advance.”
McCarroll and his colleagues then identified markers of the astrocytes’ and neurons’ contributions to SNAP, called SNAP-a and SNAP-n respectively. In an analysis of 179,764 astrocytes and 75,929 glutamatergic neurons, both SNAP-a and SNAP-n declined in the brains of people with schizophrenia and in cells from older people.