Novel neurons upend ‘yin-yang’ model of hunger, satiety in brain
The new type of leptin-sensitive cells curb hunger quickly—adding to an increasingly complex picture of brain circuits that control feeding behaviors.
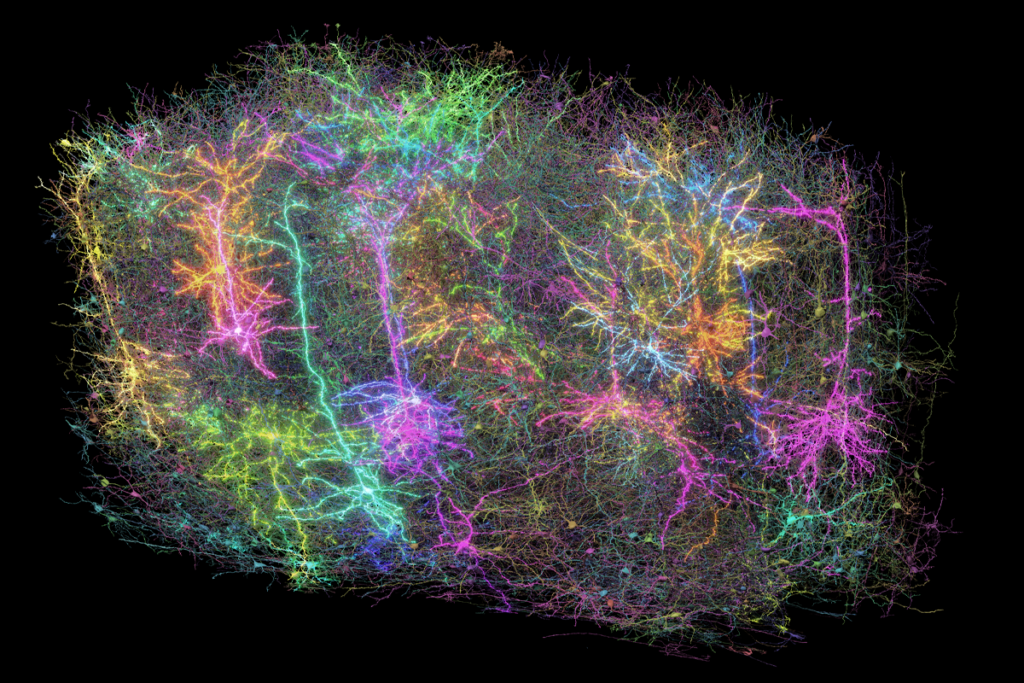
For years, scientists have thought of hunger regulation as a tug-of-war between two types of neurons in the hypothalamus: those that express the AGRP gene and increase hunger, and those that express the POMC gene and act as a brake. Now a new study challenges this long-standing model, revealing a third player in the hunger-satiety network—a neuron type that expresses the BNC2 gene and suppresses hunger faster than those that express POMC.
These BNC2 neurons are activated by leptin—a hormone that helps suppress appetite and boost metabolism. Their discovery “reshapes our understanding of feeding behavior,” says lead investigator Han Tan, “and how leptin regulates body weight.” Tan is a research associate in Jeffrey Friedman’s lab at Rockefeller University.
“We’ve known for a long time there must be [other] neurons in the brain that are sensing leptin and decreasing appetite, but we didn’t know who they were until now,” says John Campbell, assistant professor of biology at the University of Virginia, who wasn’t involved in the study.
The results jibe with two other recent reports of leptin-sensitive neurons in the arcuate nucleus—a region in the hypothalamus that processes signals related to hunger and satiety. The neurons generate feelings of fullness, an independent team reported in Science in June, and they dampen appetite by inhibiting AGRP-expressing “hunger neurons,” according to a study Campbell and his colleagues published in Nature Metabolism in December 2024.
The studies all point to a unique group of neurons that inhibit hunger, says Martin Myers, professor of internal medicine and molecular and integrative physiology at the University of Michigan, who was not involved in the work. “The three groups essentially found [these neurons] simultaneously.”
I
nterest in leptin-sensitive neurons began about 30 years ago, when Friedman and his colleagues discovered the leptin hormone. Produced by fat cells, leptin informs the brain about energy stores in the body, and mice with mutations in the leptin gene or its receptor become severely obese.Until recently, AGRP and POMC neurons were considered leptin’s main targets. But activating AGRP neurons quickly triggers appetite, whereas POMC neurons take hours to suppress hunger. That gap suggested something was missing, Tan says.
To look for other cells that express leptin receptors in mice, Tan and his colleagues turned to a technique that measures gene expression by sequencing RNA from individual nuclei. The BNC2 neurons they identified activate within seconds after a fasted mouse sees food and eventually inhibit AGRP neurons, promoting satiety. The researchers published their findings in Nature in October.
“AGRP and BNC2 neurons are the yin-yang for hunger and satiety,” Tan says, whereas POMC neurons likely play a role in the long-term regulation of body weight.
“It took a little bit of fancy footwork and the application of a fair amount of technology to identify [BNC2] neurons,” Myers says. The cells also express the GLP-1 receptor—a target of popular weight-loss drugs such as semaglutide (commonly marketed as Ozempic)—and could be important for pharmacological interventions to regulate appetite, he says. But, he adds, it’s unclear whether these neurons contribute to the daily regulation of food intake or if they’re important only during times of extreme hunger, such as in fasted animals.
Another key question is which genes in these neurons are involved in leptin’s ability to reduce appetite, Campbell says. “Now that we know the specific cells on which leptin is acting, we can answer that question.”
T
he discovery of BNC2 neurons is part of a larger effort to understand how different brain circuits control feeding behaviors. In another study published in October, Friedman’s team discovered a three-neuron circuit in the brain that links hunger signals to chewing—a process critical for eating. The circuit, which involves hypothalamic neurons that express brain-derived neurotrophic factor (BDNF), appears to control the movement of the jaw muscles. Activating BDNF neurons in mice reduces both hunger and pleasure-driven eating, whereas inhibiting them causes the animals to chew compulsively, even when no food is present.“Now we have a circuit, and we can go from the neurons that sense the energy state to the neurons that control the muscles,” says lead investigator Christin Kosse, a research associate in Friedman’s lab.
Both studies from Friedman’s team highlight the complexity of the brain circuits that control feeding behavior, says Richard Simerly, professor of molecular physiology and biophysics at Vanderbilt University, who was not involved in the studies. Hunger is not an “on or off” state but a graded response shaped by leptin and other internal signals, as well as external cues such as the presence of food, he says. So some of the findings may also help neuroscientists understand how the brain balances hunger with competing drives, such as thirst and pain.
BNC2 neurons integrate signals from both leptin and GLP-1 and may serve as key players in processing and balancing various types of information related to feeding behavior, Campbell says. “Are [BNC2 neurons] just carrying hormonal information to AGRP neurons, or are they relaying other kinds of information, like sensory information?” he says. “I think this [work] raises a lot of really exciting questions, and we now know where to look for the answers.”
Recommended reading

Should I stay (and eat) or should I go? How the brain balances hunger with competing drives

Split gene therapy delivers promise in mice modeling Dravet syndrome

U.S. human data repositories ‘under review’ for gender identity descriptors
Explore more from The Transmitter

Functional MRI can do more than you think