The cognitive decline that prompts a diagnosis of Alzheimer’s disease is like the smoke that signals a raging fire: by the time it is apparent, the damage has already begun.
That damage includes the accumulation of beta-amyloid plaques and tau tangles that kill off excitatory neurons in the hippocampus, entorhinal cortex and, eventually, other parts of the brain. But how this cascade kicks off—and how to halt its progression—remains a mystery.
Now, two single-cell brain atlases, one described last week in Nature Neuroscience and the other in Nature in August, offer a fresh glimpse at how the disease may advance. The teams behind the atlases examined postmortem samples along a continuum of Alzheimer’s progression and used single-nucleus RNA sequencing to estimate the cellular steps that accompany plaques and tangles—namely, changes in glia and the loss of certain inhibitory cells. The work follows the publication of two single-cell atlases last year that also described changes to astrocytes, microglia and inhibitory cells early in Alzheimer’s, after constructing a trajectory of the disease.
Previous RNA sequencing work looked at a single timepoint in people with a diagnosis. The new approach makes it possible to explore how cell changes over time could contribute to the disease, “in [a] much more precise manner,” says Vivek Swarup, associate professor of neurobiology and behavior at the University of California, Irvine, who was not involved in either new study.
And whereas earlier studies looked at up to about 50 brains, the latest crop of work has larger sample sizes, sometimes in the hundreds. “These are the sample size numbers that we need to really disentangle all the different types of changes in the cell types that can happen,” says Vilas Menon, assistant professor of neurological sciences at Columbia University, who co-led the new Nature study.
R
NA sequencing of single cells or nuclei can point to the specific cell types that are affected in Alzheimer’s disease, says Kyle Travaglini, scientist at the Allen Institute for Brain Science. “For more than 100 years now, the field has been very focused on proteinopathies,” such as amyloid plaques and tau tangles. But the cells those proteins affect, he says, may be better therapeutic targets than the proteins themselves have proved to be.One of the first analyses of this type in Alzheimer’s disease, published in 2019, revealed atypical expression of transcripts related to myelination in the brains of 24 people who had high levels of amyloid beta compared with 24 people who had typical levels. The following year, a different group of researchers identified a subtype of microglia that was altered in the brains of 14 people with the disease.
Whereas these past studies relied on cross-sectional data, the new generation of atlases, including the two reported last year, borrow a bioinformatics tool from developmental biology, called a pseudo-timeline: An algorithm orders the samples along an Alzheimer’s trajectory, which the teams can then use to evaluate how the transcripts changed as the disease progressed.
For the study published last week, Travaglini and his colleagues analyzed single-nucleus data from the middle temporal gyrus, where the spread of tau and amyloid first overlaps, in the brains of 84 people who ranged from having no cognitive symptoms to showing severe cognitive decline. The team quantified the levels of plaques and phosphorylated tau present in each sample using machine learning and then used that information to order the samples along a pseudo-timeline. And in the August study, Menon and his colleagues examined samples from the dorsolateral prefrontal cortex, another site of Alzheimer’s-related dysfunction, of 437 people with various cognitive symptoms, and organized their samples based on the cell subtypes they contained.
In the early stages of both pseudo-timelines, before excitatory cells began to die off, microglia and astrocytes showed signs of increased inflammation.
“What I think is very exciting here is that these very different algorithmic approaches actually result in similar biology and insights about Alzheimer’s,” says Naomi Habib, assistant professor of neuroscience at the Hebrew University of Jerusalem, who co-led the August study.
The inflammation signal came as no surprise, she adds, but the order in which these changes seem to happen was unexpected. Causal mediation modeling of the data showed a link between the inflammatory response of one microglia subgroup and an increase in plaques, and a connection between the activation of another microglia subgroup and the progression from plaques to tangles. From there, astrocytes mediated a connection between increased tangles and cognitive decline. That means, she says, that this inflammation is not just “collateral damage” but a critical step needed for Alzheimer’s to progress.
The finding points to the importance of looking beyond neuronal cell death to understand the root cause of Alzheimer’s, says Bérénice Benayoun, associate professor of gerontology at the University of Southern California, who was not involved in the new studies. “The neuron issues seem to be pretty late in the disease,” she says.
T
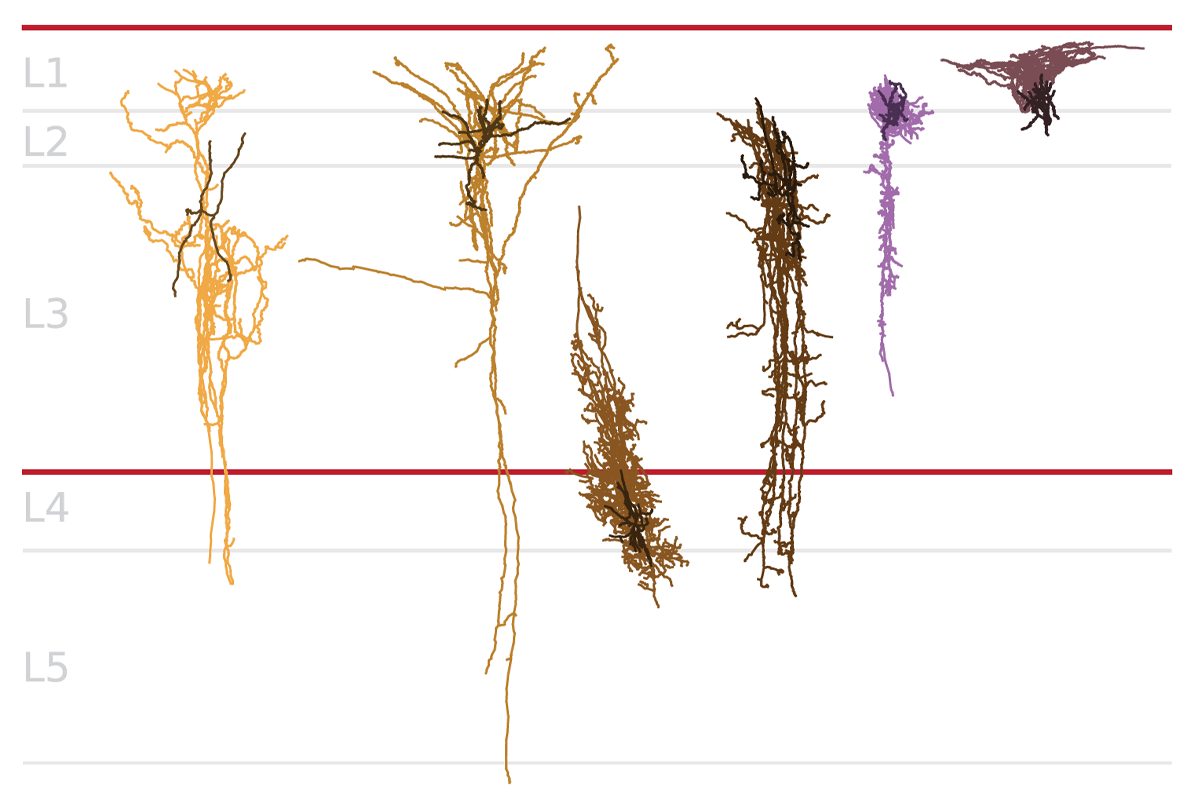
hat said, some subsets of neurons are lost early in the Alzheimer’s trajectory, both new studies show: Certain inhibitory interneurons that express somatostatin (SST), for example, are atypically low in abundance even before an exponential buildup of plaques or tangles, and potentially decades before a person would show cognitive signs of the disease.These SST inhibitory cells are “a critical element” to maintain homeostasis within neuronal circuits, says Mariano Gabitto, assistant investigator at the Allen Institute and a researcher on last week’s study. The new works point to SST cells as a potential therapeutic target in treating the disease, he says.
The idea that SST interneurons are vulnerable in Alzheimer’s is not new. In 1985, researchers identified a decrease in the concentration of somatostatin receptors in people who had been diagnosed with the disease as compared to controls. And a subset of SST interneurons are dysfunctional in a mouse model of Alzheimer’s, according to a study published in 2016.
But advances in spatial transcriptomics now reveal where in the brain these cells die off first, according to last week’s study. “Not all of the SST neurons are dying, just a subset of those” in superficial layers, Gabitto says.
Because the team behind the August study arranged their timeline based on cell subtypes, they could also delineate two separate trajectories for the aging brain: one for healthy aging and one for Alzheimer’s-related aging. In the latter trajectory, an increase in amyloid precedes an increase in tangles, which is then followed by cognitive decline; in the trajectory of healthy aging, amyloid is also present—yet the cascade is not activated.
T
he results add nuance to the much-contested amyloid hypothesis—the idea that the presence of amyloid beta in the brain is what drives neurofilament tangles and then dementia, Habib adds. Previous postmortem brain studies have shown that people without Alzheimer’s also have amyloid beta build-up in their brains that does not become pathogenic. Habib’s study suggests that it is not the amyloid itself that results in the disease, but the glial cells’ response to it, or something else that occurs in the early stages of the disease, she says. “Amyloid might be at the beginning of this cascade, but the cellular response to this cascade really shapes the fate of the brain.”Parsing these two trajectories could help future studies pinpoint signals that are Alzheimer’s specific and those that are just an effect of healthy aging, Habib says. “What we previously called ‘healthy’ probably included a combination of healthy and those who are already on a different path of aging,” which could have added noise to past analyses, she says.
Past work, including a 2011 study, found increased phosphorylated tau in excitatory cells in the early stages of the disease, says Amy Arnsten, professor of neuroscience at Yale School of Medicine, who was not involved in the studies. RNA sequencing cannot assess post-translational modifications, she adds. “I think these papers give the false impression that the excitatory cells aren’t already in an inflammatory state,” she says.
Another shortcoming is the fact that the teams did not analyze sex differences in how the disease progresses, Benayoun adds. About two-thirds of Alzheimer’s patients are women, according to data from the Alzheimer’s Association. “We also know there are fundamental differences in the way females and males age,” Benayoun says.
The teams aim to diversify their cohorts as they add more data, which should help get at how these changes differ by sex and race, Travaglini says. “We know that there are disparate effects,” he adds.
The two teams have already started combining their datasets to increase their total sample size, Travaglini says.